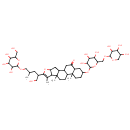| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:09:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:01 UTC |
|---|
| Accession Number | CHEM025133 |
|---|
| Identification |
|---|
| Common Name | Chinenoside V |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Allium chinense (rakkyo). Chinenoside V is found in onion-family vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 26-O-beta-Glucopyranosyl-3 beta,26-dihydroxy-23-hydroxymethyl-25(R)-5alpha-furost-20(22)-en-6-one 3-O-alpha-arabinopyranosyl(1-6)-beta-glucopyranoside | MeSH | | Chinenoside V | MeSH |
|
|---|
| Chemical Formula | C45H72O19 |
|---|
| Average Molecular Mass | 917.042 g/mol |
|---|
| Monoisotopic Mass | 916.467 g/mol |
|---|
| CAS Registry Number | 170739-22-7 |
|---|
| IUPAC Name | 6-(1-hydroxy-4-methyl-5-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}pentan-2-yl)-7,9,13-trimethyl-16-[(3,4,5-trihydroxy-6-{[(3,4,5-trihydroxyoxan-2-yl)oxy]methyl}oxan-2-yl)oxy]-5-oxapentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icos-6-en-19-one |
|---|
| Traditional Name | 6-(1-hydroxy-4-methyl-5-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}pentan-2-yl)-7,9,13-trimethyl-16-[(3,4,5-trihydroxy-6-{[(3,4,5-trihydroxyoxan-2-yl)oxy]methyl}oxan-2-yl)oxy]-5-oxapentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icos-6-en-19-one |
|---|
| SMILES | CC(COC1OC(CO)C(O)C(O)C1O)CC(CO)C1=C(C)C2C(CC3C4CC(=O)C5CC(CCC5(C)C4CCC23C)OC2OC(COC3OCC(O)C(O)C3O)C(O)C(O)C2O)O1 |
|---|
| InChI Identifier | InChI=1S/C45H72O19/c1-18(15-58-42-38(56)35(53)33(51)29(14-47)63-42)9-20(13-46)40-19(2)31-28(62-40)12-24-22-11-26(48)25-10-21(5-7-44(25,3)23(22)6-8-45(24,31)4)61-43-39(57)36(54)34(52)30(64-43)17-60-41-37(55)32(50)27(49)16-59-41/h18,20-25,27-39,41-43,46-47,49-57H,5-17H2,1-4H3 |
|---|
| InChI Key | WDTQKOJLOMDSGJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroidal saponins. These are saponins in which the aglycone moiety is a steroid. The steroidal aglycone is usually a spirostane, furostane, spirosolane, solanidane, or curcubitacin derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroidal saponins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052k-0200219571-575f5da63f51b8cd2411 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0200509410-36c1e05bf4ee355d2739 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0btc-3615709020-88f11d19baaa50b94c52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fr2-1920006465-8b23f27c165801f2cfea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-007k-3900115140-b90f97fe70e7a3913d87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006y-8900256010-c039049ca71a61b6f8fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0600000259-78f67b01b34fdb8ca7af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066u-6000001961-b2875bc09d2deeb0b3c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0adl-8100000930-389421a45e4f3aff6156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0100002912-b486edce504856dd9fef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-0100426902-46af1b1f10352e8fd432 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014r-7700084921-58eb5f895a4ab8cb262c | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031139 |
|---|
| FooDB ID | FDB003151 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024133 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751143 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|