| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:09:30 UTC |
|---|
| Update Date | 2016-11-09 01:18:01 UTC |
|---|
| Accession Number | CHEM025128 |
|---|
| Identification |
|---|
| Common Name | Neoacrimarine G |
|---|
| Class | Small Molecule |
|---|
| Description | Neoacrimarine G is found in citrus. Neoacrimarine G is an alkaloid from the roots of Citrus paradisi (grapefruit |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
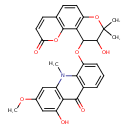
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Neoacrimarine-g | HMDB |
|
|---|
| Chemical Formula | C29H25NO8 |
|---|
| Average Molecular Mass | 515.511 g/mol |
|---|
| Monoisotopic Mass | 515.158 g/mol |
|---|
| CAS Registry Number | 195057-37-5 |
|---|
| IUPAC Name | 1-hydroxy-5-({13-hydroxy-12,12-dimethyl-4-oxo-3,11-dioxatricyclo[8.4.0.0²,⁷]tetradeca-1(10),2(7),5,8-tetraen-14-yl}oxy)-3-methoxy-10-methyl-9,10-dihydroacridin-9-one |
|---|
| Traditional Name | 1-hydroxy-5-({13-hydroxy-12,12-dimethyl-4-oxo-3,11-dioxatricyclo[8.4.0.0²,⁷]tetradeca-1(10),2(7),5,8-tetraen-14-yl}oxy)-3-methoxy-10-methylacridin-9-one |
|---|
| SMILES | COC1=CC2=C(C(O)=C1)C(=O)C1=C(N2C)C(OC2C(O)C(C)(C)OC3=C2C2=C(C=CC(=O)O2)C=C3)=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C29H25NO8/c1-29(2)28(34)27(23-19(38-29)10-8-14-9-11-21(32)37-26(14)23)36-20-7-5-6-16-24(20)30(3)17-12-15(35-4)13-18(31)22(17)25(16)33/h5-13,27-28,31,34H,1-4H3 |
|---|
| InChI Key | MNOCITGIWBOSNH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-0090610000-1b40f47235745c44557d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014i-1006019000-fc04f145782f63ad7886 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000490000-05d6258aeb63b57ac67a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ke-1010930000-b78206bae9cd61d86fc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-057i-0390200000-d78b82653aca3e134acb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0010490000-6bf974e7766a401b640b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-022c-1031930000-b8af0118bf9e38065425 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-1190000000-22c80c38c8c7bad401d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-bc0300cdff368f6a084c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0130960000-0cbd2b220cc3c8ed63c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03kc-0350900000-502cf67401bb8a999789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000090000-928cf2595cb11ecbe433 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0021690000-f28cec02d31dcfa6792a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0hbi-1591510000-9140291a18798092cdc7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031135 |
|---|
| FooDB ID | FDB003146 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024225 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013323 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751141 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|