| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:05:58 UTC |
|---|
| Update Date | 2016-11-09 01:18:00 UTC |
|---|
| Accession Number | CHEM025042 |
|---|
| Identification |
|---|
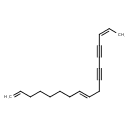
| Common Name | (8E,15E)-1,8,15-Heptadecatriene-11,13-diyne |
|---|
| Class | Small Molecule |
|---|
| Description | (8Z,15Z)-1,8,15-Heptadecatriene-11,13-diyne is found in coffee and coffee products. (8Z,15Z)-1,8,15-Heptadecatriene-11,13-diyne occurs in Silybum marianum (milk thistle |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Triazolopyrimidine-based compound, DSM121 | HMDB |
|
|---|
| Chemical Formula | C17H22 |
|---|
| Average Molecular Mass | 226.357 g/mol |
|---|
| Monoisotopic Mass | 226.172 g/mol |
|---|
| CAS Registry Number | 28834-02-8 |
|---|
| IUPAC Name | (8E,15Z)-heptadeca-1,8,15-trien-11,13-diyne |
|---|
| Traditional Name | (8E,15Z)-heptadeca-1,8,15-trien-11,13-diyne |
|---|
| SMILES | C\C=C/C#CC#CC\C=C\CCCCCC=C |
|---|
| InChI Identifier | InChI=1S/C17H22/c1-3-5-7-9-11-13-15-17-16-14-12-10-8-6-4-2/h3-4,6,15,17H,1,5,7,9,11,13,16H2,2H3/b6-4-,17-15+ |
|---|
| InChI Key | OJWVHJFAQCYGMT-LPMQCFACSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as enynes. These are hydrocarbons containing an alkene and an alkyne group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Enynes |
|---|
| Direct Parent | Enynes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Enyne
- Unsaturated aliphatic hydrocarbon
- Olefin
- Acyclic olefin
- Acyclic acetylene
- Acetylene
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05nf-5900000000-0dc224b10bb5585ee93a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0190000000-16fee79dc4a2cb258c4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06vi-5950000000-29bfabfa1056f36b2fc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-9500000000-b6fcca4d3792241bf6ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-31bc321c2ebb275f3937 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0290000000-2adf1d88b8ab1b92be67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f79-4910000000-a29e8f876ad8c0709536 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-7790000000-21810ab81171f07d18cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-009i-9300000000-5fab75cf182e0448a316 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004r-9200000000-38a6903fc6bd084d3c94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-1f4b5e9f4041e76305f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0490000000-290fa9f6750bd7ce0911 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0292-5910000000-f742f511cfb768168ff6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031041 |
|---|
| FooDB ID | FDB003035 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776874 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751122 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|