| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:02:51 UTC |
|---|
| Update Date | 2016-11-09 01:17:59 UTC |
|---|
| Accession Number | CHEM024967 |
|---|
| Identification |
|---|
| Common Name | (2E,4E)-Decadienoic isobutylamide |
|---|
| Class | Small Molecule |
|---|
| Description | (E,E)-2,4-Decadienoic isobutylamide is found in herbs and spices. (E,E)-2,4-Decadienoic isobutylamide is a constituent of Achillea millefolium (yarrow) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
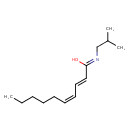
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E)-N-Isobutyl-2,4-decadienamide | HMDB | | (e,e)-N-(2-Methylpropyl)-2,4-decadienamide | HMDB | | (e,e)-N-Isobutyl-2,4-decadienamide | HMDB | | N-(2-Methylpropyl)-(2E,4E)-2,4-decadienamide | HMDB | | N-(2-Methylpropyl)-(e,e)-2,4-decadienamide | HMDB | | N-(2-Methylpropyl)-2,4-decadienamide | HMDB | | N-Isobutyl-(e,e)-2,4-decadienamide | HMDB | | N-Isobutyldeca-trans-2,trans-4-dienamide | HMDB | | N-Isobutyldeca-trans-2-trans-4-dienamide | HMDB | | Pellitorin | HMDB | | Pellitorine | HMDB | | Pellitorine (6ci) | HMDB | | trans-Pellitorin | HMDB |
|
|---|
| Chemical Formula | C14H25NO |
|---|
| Average Molecular Mass | 223.354 g/mol |
|---|
| Monoisotopic Mass | 223.194 g/mol |
|---|
| CAS Registry Number | 18836-52-7 |
|---|
| IUPAC Name | (Z,2E,4Z)-N-(2-methylpropyl)deca-2,4-dienimidic acid |
|---|
| Traditional Name | (Z,2E,4Z)-N-(2-methylpropyl)deca-2,4-dienimidic acid |
|---|
| SMILES | CCCCC\C=C/C=C/C(/O)=N/CC(C)C |
|---|
| InChI Identifier | InChI=1S/C14H25NO/c1-4-5-6-7-8-9-10-11-14(16)15-12-13(2)3/h8-11,13H,4-7,12H2,1-3H3,(H,15,16)/b9-8-,11-10+ |
|---|
| InChI Key | MAGQQZHFHJDIRE-QNRZBPGKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl amines. N-acyl amines are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty amides |
|---|
| Direct Parent | N-acyl amines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-amine
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pb9-8900000000-5a3ffabd7640b8a20589 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0ab9-6190000000-f5116065ce93702750dd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9130000000-7fae23a78251bf7bd4f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9100000000-c9953cd30fb48a569729 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-3cc24bd9ddb8e27f4a4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1390000000-98c5657fd3011747d0ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-4940000000-5a8b547d26e6bacc9226 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-9800000000-223c45153be25f9bb861 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0190000000-99a9162b378cec1750bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-7980000000-53fc5f0ac0f6e901fbfa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-9300000000-dca7a7c316587d2950ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-5590000000-9be25e5a3ad24d5ae560 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-479d2bb1e9e64618908d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-a6873ff8e03899bc15e0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030951 |
|---|
| FooDB ID | FDB002929 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00028813 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9542995 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11368078 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|