| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:02:15 UTC |
|---|
| Update Date | 2016-11-09 01:17:59 UTC |
|---|
| Accession Number | CHEM024953 |
|---|
| Identification |
|---|
| Common Name | Triglochinin |
|---|
| Class | Small Molecule |
|---|
| Description | Isotriglochinin is found in green vegetables. Isotriglochinin is a constituent of the famine food Alocasia macrorrhiza (wild taro). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
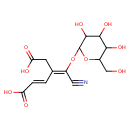
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4E)-4-[Cyano({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})methylidene]hex-2-enedioate | HMDB | | Triglochinin | MeSH |
|
|---|
| Chemical Formula | C14H17NO10 |
|---|
| Average Molecular Mass | 359.286 g/mol |
|---|
| Monoisotopic Mass | 359.085 g/mol |
|---|
| CAS Registry Number | 28876-11-1 |
|---|
| IUPAC Name | (2E,4E)-4-[cyano({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})methylidene]hex-2-enedioic acid |
|---|
| Traditional Name | (2E,4E)-4-[cyano({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})methylidene]hex-2-enedioic acid |
|---|
| SMILES | OCC1OC(O\C(C#N)=C(/CC(O)=O)\C=C\C(O)=O)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C14H17NO10/c15-4-7(6(3-10(19)20)1-2-9(17)18)24-14-13(23)12(22)11(21)8(5-16)25-14/h1-2,8,11-14,16,21-23H,3,5H2,(H,17,18)(H,19,20)/b2-1+,7-6- |
|---|
| InChI Key | LABCALMTQNDOAI-RPRRTJJHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyanogenic glycosides. These are glycosides in which the aglycone moiety contains a cyanide group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Cyanogenic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyanogenic glycoside
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- Hexose monosaccharide
- O-glycosyl compound
- Medium-chain fatty acid
- Amino fatty acid
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Fatty acid
- Fatty acyl
- Unsaturated fatty acid
- Dicarboxylic acid or derivatives
- Oxane
- Monosaccharide
- Secondary alcohol
- Organoheterocyclic compound
- Acetal
- Carboxylic acid derivative
- Carboxylic acid
- Polyol
- Oxacycle
- Nitrile
- Carbonitrile
- Organic nitrogen compound
- Primary alcohol
- Organopnictogen compound
- Organic oxide
- Organonitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-7839000000-b68ec06cb0de7100542b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-001i-3601169000-c047dd1c8eca8816f80b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01x4-0819000000-36e1be9775e10a07d61a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uea-0900000000-080670c05240455572aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-1900000000-2cf804b3f619fc6ae590 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-1729000000-720255b4ac45137ce6f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-1912000000-822dcd3b29cdfac1ff44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udv-3900000000-6d3a53f7de16cddb735a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0209000000-46e2a1f596b6856f04e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ed-0958000000-fd43f409f91bd5411383 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0079-2920000000-89d4e095835c67deb935 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0972000000-1955672129be8cc2be8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0690000000-1d3cea022233a8169fcd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1900000000-92633d898c6b2ba3d522 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030934 |
|---|
| FooDB ID | FDB002903 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001457 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 11671601 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6088720 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|