| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:59:12 UTC |
|---|
| Update Date | 2016-11-09 01:17:58 UTC |
|---|
| Accession Number | CHEM024881 |
|---|
| Identification |
|---|
| Common Name | Austdiol |
|---|
| Class | Small Molecule |
|---|
| Description | Austdiol is a toxic metabolite of the food storage mould Aspergillus ustu |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
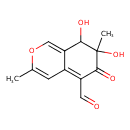
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (7R,8S)-7,8-Dihydroxy-3,7-dimethyl-6-oxo-7,8-dihydro-6H-isochromene-5-carbaldehyde | HMDB | | 7,8-Dihydro-7,8-dihydroxy-3,7-dimethyl-6-oxo-6H-2-benzopyran-5-carboxaldehyde, 9ci | HMDB | | Austadiol | HMDB | | Austidiol | HMDB | | Austdiol | MeSH |
|
|---|
| Chemical Formula | C12H12O5 |
|---|
| Average Molecular Mass | 236.221 g/mol |
|---|
| Monoisotopic Mass | 236.068 g/mol |
|---|
| CAS Registry Number | 53043-28-0 |
|---|
| IUPAC Name | 7,8-dihydroxy-3,7-dimethyl-6-oxo-7,8-dihydro-6H-isochromene-5-carbaldehyde |
|---|
| Traditional Name | 7,8-dihydroxy-3,7-dimethyl-6-oxo-8H-isochromene-5-carbaldehyde |
|---|
| SMILES | CC1=CC2=C(C=O)C(=O)C(C)(O)C(O)C2=CO1 |
|---|
| InChI Identifier | InChI=1S/C12H12O5/c1-6-3-7-8(4-13)10(14)12(2,16)11(15)9(7)5-17-6/h3-5,11,15-16H,1-2H3 |
|---|
| InChI Key | QVMUHZHZYCDMAI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as azaphilones. These are a structurally variable family of fungal polyketide metabolites possessing a highly oxygenated pyranoquinone bicyclic core, usually known as isochromene, and a quaternary carbon center. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azaphilones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Azaphilones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Azaphilone
- Cyclohexenone
- Acyloin
- Pyran
- Tertiary alcohol
- 1,2-diol
- Ketone
- Cyclic ketone
- Secondary alcohol
- Oxacycle
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Aldehyde
- Alcohol
- Organic oxide
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bt9-1940000000-2660c21588222ac74cb2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0i90-8219000000-a4b2c393d631717a52bb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-11f669c5fb0bfcdc3807 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014s-0390000000-e1440e6636fc94c5a17d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9810000000-91e2542326e884354aa9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0190000000-d06ccc75f498ebead345 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-2690000000-f56c13b73508235f2b56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052u-3910000000-ebf498c8df190cc123e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-3c24cbdfc0b9419176cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q0-3950000000-f724c6f16ba94cff491c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-1900000000-3c323e16ce4e4e3401c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-521ee4207139663f4aba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ko-0790000000-9188a0d044f9b942dd45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9600000000-210f2183ed222a578eb9 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030858 |
|---|
| FooDB ID | FDB002818 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00052851 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2759545 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3520232 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|