| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:58:34 UTC |
|---|
| Update Date | 2016-11-09 01:17:58 UTC |
|---|
| Accession Number | CHEM024865 |
|---|
| Identification |
|---|
| Common Name | 4',5,7-Trimethylapigenin |
|---|
| Class | Small Molecule |
|---|
| Description | 4',5,7-Trimethoxyflavone, also known as trimethylapigenin, belongs to the class of organic compounds known as 7-O-methylated flavonoids. These are flavonoids with methoxy groups attached to the C7 atom of the flavonoid backbone. Thus, 4',5,7-trimethoxyflavone is considered to be a flavonoid lipid molecule. 4',5,7-Trimethoxyflavone is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Outside of the human body, 4',5,7-trimethoxyflavone has been detected, but not quantified in, a few different foods, such as citrus, mandarin orange (clementine, tangerine), and sweet oranges. This could make 4',5,7-trimethoxyflavone a potential biomarker for the consumption of these foods. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
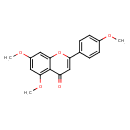
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4',5,7-Trimethyl-apigenin | MeSH | | 5,7,4'-Trimethylapigenin | MeSH | | 4'57-Trimethoxyflavone | ChEMBL, HMDB | | 4',5,7-Trimethoxy-flavone | HMDB | | 5,7,4'-Trimethoxyflavone | HMDB | | 5,7-Dimethoxy-2-(4-methoxyphenyl)-4H-1-benzenopyran-4-one | HMDB | | 5,7-Dimethoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one | HMDB | | 6-Hydroxy-4',5,7-trimethoxyflavone | HMDB | | Apigenin 5,7,4'-trimethyl ether | HMDB | | Apigenin trimethyl ether | HMDB | | 4',5,7-Trimethoxyflavone | MeSH | | 4’,5,7-Trimethoxyflavone | HMDB | | 5,7,4'-Tri-O-methylapigenin | HMDB | | 5,7,4'-Trimethoxyapigenin | HMDB | | 5,7,4’-Tri-O-methylapigenin | HMDB | | 5,7,4’-Trimethoxyapigenin | HMDB | | 5,7,4’-Trimethoxyflavone | HMDB | | 5,7-Dimethoxy-2-(4-methoxyphenyl)-4H-chromen-4-one | HMDB | | 5,7-Dimethoxy-2-(4-methoxyphenyl)chromone | HMDB | | 5,7-Dimethoxy-4-oxo-2-(4-methoxyphenyl)-4H-1-benzopyran | HMDB | | Tri-O-methylapigenin | HMDB | | Trimethylapigenin | HMDB |
|
|---|
| Chemical Formula | C18H16O5 |
|---|
| Average Molecular Mass | 312.317 g/mol |
|---|
| Monoisotopic Mass | 312.100 g/mol |
|---|
| CAS Registry Number | 5631-70-9 |
|---|
| IUPAC Name | 5,7-dimethoxy-2-(4-methoxyphenyl)-4H-chromen-4-one |
|---|
| Traditional Name | 5,7-dimethoxy-2-(4-methoxyphenyl)chromen-4-one |
|---|
| SMILES | COC1=CC=C(C=C1)C1=CC(=O)C2=C(OC)C=C(OC)C=C2O1 |
|---|
| InChI Identifier | InChI=1S/C18H16O5/c1-20-12-6-4-11(5-7-12)15-10-14(19)18-16(22-3)8-13(21-2)9-17(18)23-15/h4-10H,1-3H3 |
|---|
| InChI Key | ZXJJBDHPUHUUHD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 7-o-methylated flavonoids. These are flavonoids with methoxy groups attached to the C7 atom of the flavonoid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | O-methylated flavonoids |
|---|
| Direct Parent | 7-O-methylated flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4p-methoxyflavonoid-skeleton
- 5-methoxyflavonoid-skeleton
- 7-methoxyflavonoid-skeleton
- Flavone
- Chromone
- Benzopyran
- 1-benzopyran
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Monocyclic benzene moiety
- Pyran
- Vinylogous ester
- Heteroaromatic compound
- Ether
- Organoheterocyclic compound
- Oxacycle
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01qa-0691000000-7a823829ea08d5c44987 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-5c2fa314b535098da3c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-e66ded4021486610bfbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-1490000000-a1ba798957d115c766da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-6fd59ab6ee43b4030a5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0029000000-1598e4635c488fb5e524 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kcr-1390000000-6872b99524899c8fbf57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-0c9c5e8761c67e87c624 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0019000000-d5759b18375da517980a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0g4j-0190000000-5d008847ec007268d4ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-70f2c2cfe29137281455 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xs-0097000000-951d276ce9092ed6732e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030842 |
|---|
| FooDB ID | FDB002800 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003821 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 72029 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 79730 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|