| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:58:10 UTC |
|---|
| Update Date | 2016-11-09 01:17:58 UTC |
|---|
| Accession Number | CHEM024855 |
|---|
| Identification |
|---|
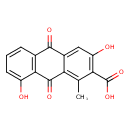
| Common Name | 3,8-Dihydroxy-1-methylanthraquinone-2-carboxylic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 3,8-Dihydroxy-1-methylanthraquinone-2-carboxylic acid is found in saffron. 3,8-Dihydroxy-1-methylanthraquinone-2-carboxylic acid is a constituent of Crocus sativus (saffron) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,8-Dihydroxy-1-methylanthraquinone-2-carboxylate | Generator | | 9,10-dihydro-3,8-Dihydroxy-1-methyl-9,10-dioxo-2-anthracenecarboxylic acid, 9ci | HMDB | | 3,8-Dihydroxy-1-methyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate | Generator | | 3,8-Dihydroxy-1-methylanthraquinone-2-carboxylic acid | MeSH | | DMAC-3,8 | MeSH | | 3,8-Dihydroxymethylanthraquinone carboxylic acid | MeSH |
|
|---|
| Chemical Formula | C16H10O6 |
|---|
| Average Molecular Mass | 298.247 g/mol |
|---|
| Monoisotopic Mass | 298.048 g/mol |
|---|
| CAS Registry Number | 69119-31-9 |
|---|
| IUPAC Name | 3,8-dihydroxy-1-methyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid |
|---|
| Traditional Name | 3,8-dihydroxy-1-methyl-9,10-dioxoanthracene-2-carboxylic acid |
|---|
| SMILES | CC1=C2C(=O)C3=C(C=CC=C3O)C(=O)C2=CC(O)=C1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C16H10O6/c1-6-11-8(5-10(18)12(6)16(21)22)14(19)7-3-2-4-9(17)13(7)15(11)20/h2-5,17-18H,1H3,(H,21,22) |
|---|
| InChI Key | MHABMANUFPZXEB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthracenecarboxylic acids. These are organic compounds containing a carboxylic acid group attached to an anthracene ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthracenecarboxylic acids and derivatives |
|---|
| Direct Parent | Anthracenecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthracene carboxylic acid
- 9,10-anthraquinone
- Anthraquinone
- Hydroxyanthraquinone
- 2-naphthalenecarboxylic acid or derivatives
- 2-naphthalenecarboxylic acid
- Salicylic acid or derivatives
- Hydroxybenzoic acid
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Vinylogous acid
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fir-0490000000-125a9b2d8ba2e52ead02 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00dv-6203910000-d048503afe44baea50b6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-4031c6b271db004a5a40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-0090000000-2b96f4544e6255b71ffc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0019-2090000000-cc3c1d000960bbd7f1fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0090000000-8eed3311a06a79a4ee91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-ea32876963f7732a65cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1090000000-da28f58982d0c3ae4e75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0090000000-4b231a8cd56f86d5ee83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-a1af9499e0121d9c7efe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0390000000-17eb57f61753259990f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0090000000-ff57c6adc82fbe6db529 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-941f00f78ae70af18204 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ufr-0090000000-7345e0ee16c974005862 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030830 |
|---|
| FooDB ID | FDB002786 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055494 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 11297920 |
|---|
| ChEBI ID | 174833 |
|---|
| PubChem Compound ID | 14379528 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|