| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:57:27 UTC |
|---|
| Update Date | 2016-11-09 01:17:58 UTC |
|---|
| Accession Number | CHEM024835 |
|---|
| Identification |
|---|
| Common Name | Porson |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Myrica gale (bog myrtle)
Gibbon's verdict on the book, that it was "the most acute and accurate piece of criticism since the days of Bentley," may be considered as somewhat partial, as it was in defence of him that Porson had entered the field against Travis. But in the masterly sketch of Gibbon's work and style in the preface Porson does not write in a merely flattering tone. It is to be wished that on such a subject the tone of levity had been modified. But Porson says in his preface that he could treat the subject in no other manner, if he treated it at all: "To peruse such a mass of falsehood and sophistry and to write remarks upon it, without sometimes giving way to laughter and sometimes to indignation, was, to me at least, impossible." Travis has no mercy shown him, but he certainly deserved none. One is equally struck with the thorough grasp Porson displays of his subject, the amount of his miscellaneous learning, and the humour that pervades the whole. But it was then the unpopular side: the publisher is said to have lost money by the book; and one of his early friends, Mrs Turner of Norwich, cut down a legacy she had left Porson to £30 on being told that he had written what was described to her as a book against Christianity.; His library was divided into two parts, one of which was sold by auction; the other, containing the transcript of the Gale Photius, his books with his notes, and some letters from foreign scholars, was bought by Trinity College for 1000 guineas. His notebooks were found to contain, in the words of Bishop Blomfield, "a rich treasure of criticism in every branch of classical literature?everything carefully and correctly written and sometimes rewritten?quite fit to meet the public eye, without any diminution or addition." They have been carefully rearranged, and illustrate among other things his extraordinary penmanship and power of minute and accurate writing. Much remains unpublished. J. H. Monk, his successor as Greek professor, and C. J. Blomfield (both afterwards bishops) edited the Adversaria, consisting of the notes on Athenaeus and the Greek poets, and his prelection on Euripides; PP Dobree, afterwards Greek professor, the notes on Aristophanes and the lexicon of Photius. Besides these, from other sources, Professor T Gaisford edited his notes on Pausanias and Suidas, and Mr Kidd collected his scattered reviews. And, when Bishop Burgess attacked his literary character on the score of his Letters to Travis, Professor Turton (afterwards Bishop of Ely) came forward with a vindication.; In 1792 his fellowship was no longer tenable by a layman; and, rather than undertake duties for which he felt himself unfit, and which involved subscription to the Articles (though he had no difficulty as to signing a statement as to his conformity with the liturgy of the Church of England when elected Greek professor), he determined not to take holy orders, which would have enabled him to remain a fellow, and thus deprived himself of his only means of subsistence. He might have been retained in the society by being appointed to a lay fellowship, one of the two permanent lay fellowships which the statutes then permitted falling vacant just in time. It is said that this had been promised him, and it was certainly the custom in the college always to appoint the senior among the existing laymen, who otherwise would vacate his fellowship. But the master (Dr Postlethwaite), who had the nomination, used his privilege to nominate a younger man (John Heys), a nephew of his own, and thus Porson was turned adrift without any means of support. A subscription was, however, got up among his friends to provide an annuity to keep him from actual want; Cracherode, Cleaver Banks, Burney and Parr took the lead, and enough was collected to produce about £100 a year. He accepted it only on the condition that he should receive the interest during his lifetime, and that the principal, placed in the hands of trustees, should be returned to the donors at his death. When this occurred they or their survivors refused to receive the money, and it was with part of this sum that, in 1816, the Porson prize was founded to perpetuate his name at Cambridge. The remainder was devoted to the foundation of the Porson scholarship in the same university. This scholarship was first awarded in 1855. Porson is found in herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
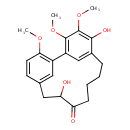
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C22H26O6 |
|---|
| Average Molecular Mass | 386.438 g/mol |
|---|
| Monoisotopic Mass | 386.173 g/mol |
|---|
| CAS Registry Number | 56222-03-8 |
|---|
| IUPAC Name | 8,15-dihydroxy-3,16,17-trimethoxytricyclo[12.3.1.1²,⁶]nonadeca-1(17),2,4,6(19),14(18),15-hexaen-9-one |
|---|
| Traditional Name | 8,15-dihydroxy-3,16,17-trimethoxytricyclo[12.3.1.1²,⁶]nonadeca-1(17),2,4,6(19),14(18),15-hexaen-9-one |
|---|
| SMILES | COC1=C2C=C(CC(O)C(=O)CCCCC3=CC2=C(OC)C(OC)=C3O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C22H26O6/c1-26-19-9-8-13-10-15(19)16-12-14(20(25)22(28-3)21(16)27-2)6-4-5-7-17(23)18(24)11-13/h8-10,12,18,24-25H,4-7,11H2,1-3H3 |
|---|
| InChI Key | VHBRVHUPCPJJMZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as meta,meta-bridged biphenyls. These are cyclic diarylheptanoids where the two aryl groups are linked to each other by an ether group conjugated to their 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Diarylheptanoids |
|---|
| Sub Class | Cyclic diarylheptanoids |
|---|
| Direct Parent | Meta,meta-bridged biphenyls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta,meta-bridged biphenyl
- Anisole
- Alkyl aryl ether
- Benzenoid
- Cyclic ketone
- Secondary alcohol
- Ketone
- Ether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0avi-0009000000-fff2fbc9cf93fda14c15 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01bi-2000950000-b7d5f3c8222074f95e6d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-b59f79f1d0b471bd0134 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-1009000000-aaee257253d9379a1f30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-4039000000-5e68ff81d2a6c71a7087 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-620dbb7953b11e7aabfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-6c786646d00bdfe6ff27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-029j-2069000000-5483085d6cc786aad5cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-37da37047cdf1c71272e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-24382024cd96ba599b5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0h2r-0029000000-8640ef752f9415d9ab37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0009000000-fa6db55a8454ab2d4923 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fri-0009000000-2014d1516dfad159036d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0059000000-d4cdb7a8da9b4d44e44b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030810 |
|---|
| FooDB ID | FDB002764 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Richard Porson |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751083 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|