| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:56:55 UTC |
|---|
| Update Date | 2016-11-09 01:17:57 UTC |
|---|
| Accession Number | CHEM024819 |
|---|
| Identification |
|---|
| Common Name | Patuletin |
|---|
| Class | Small Molecule |
|---|
| Description | A trimethoxyflavone that is quercetagetin methylated at position 6. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
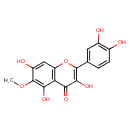
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4-benzopyrone | ChEBI | | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4H-1-benzopyran-4-one | ChEBI | | 3',4',5,7-Tetrahydroxy-6-methoxyflavonol | ChEBI | | 3,5,7,3',4'-Pentahydroxy-6-methoxyflavone | ChEBI | | 6-Methoxyquercetin | ChEBI | | 6-O-Methylquercetagetin | ChEBI | | Quercetagetin 6-methyl ether | ChEBI | | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4H-1-benzopyran-4-one, 9ci | HMDB | | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4H-chromen-4-one | MeSH |
|
|---|
| Chemical Formula | C16H12O8 |
|---|
| Average Molecular Mass | 332.262 g/mol |
|---|
| Monoisotopic Mass | 332.053 g/mol |
|---|
| CAS Registry Number | 519-96-0 |
|---|
| IUPAC Name | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4H-chromen-4-one |
|---|
| Traditional Name | patuletin |
|---|
| SMILES | COC1=C(O)C2=C(OC(=C(O)C2=O)C2=CC(O)=C(O)C=C2)C=C1O |
|---|
| InChI Identifier | InChI=1S/C16H12O8/c1-23-16-9(19)5-10-11(13(16)21)12(20)14(22)15(24-10)6-2-3-7(17)8(18)4-6/h2-5,17-19,21-22H,1H3 |
|---|
| InChI Key | JMIFIYIEXODVTO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonols. Flavonols are compounds that contain a flavone (2-phenyl-1-benzopyran-4-one) backbone carrying a hydroxyl group at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavones |
|---|
| Direct Parent | Flavonols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 6-methoxyflavonoid-skeleton
- 3-hydroxyflavone
- Hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 3-hydroxyflavonoid
- 3'-hydroxyflavonoid
- Chromone
- 1-benzopyran
- Benzopyran
- Anisole
- Catechol
- Pyranone
- Phenol
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Pyran
- Monocyclic benzene moiety
- Vinylogous acid
- Heteroaromatic compound
- Ether
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-0529000000-80eb16e79922e773ce42 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-004i-0024029000-5b6566c1e3d3b3e22ad5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-0433b24ac8fa3594d2ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0109000000-8ce46b5c40f4ed741ffd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05n0-4921000000-98be6ac7065ad4f7fb0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0109000000-0074b1f85f21aec6fa6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0139000000-11cfd247fb3babf52f64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-6941000000-01e1b2cba8f4e48697a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-9a57877c53466fab202c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0009000000-58dc8929de3f58a5353d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1903000000-74531964f65f03d51054 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-60f511470aa84a372601 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0439000000-9d8b568c9fc819b97088 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01c0-1921000000-7210a1e07ea954f6ac6a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030802 |
|---|
| FooDB ID | FDB002744 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00004680 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-14907 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Patuletin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 75164 |
|---|
| PubChem Compound ID | 5281678 |
|---|
| Kegg Compound ID | C10118 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|