| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:56:51 UTC |
|---|
| Update Date | 2016-11-09 01:17:57 UTC |
|---|
| Accession Number | CHEM024817 |
|---|
| Identification |
|---|
| Common Name | 5-Deoxymyricanone |
|---|
| Class | Small Molecule |
|---|
| Description | 5-Deoxymyricanone is found in fruits. 5-Deoxymyricanone is isolated from bacterial galls of Myrica rubra (Chinese bayberry |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
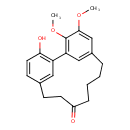
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C21H24O4 |
|---|
| Average Molecular Mass | 340.413 g/mol |
|---|
| Monoisotopic Mass | 340.167 g/mol |
|---|
| CAS Registry Number | 110007-10-8 |
|---|
| IUPAC Name | 3-hydroxy-16,17-dimethoxytricyclo[12.3.1.1²,⁶]nonadeca-1(17),2,4,6(19),14(18),15-hexaen-9-one |
|---|
| Traditional Name | 3-hydroxy-16,17-dimethoxytricyclo[12.3.1.1²,⁶]nonadeca-1(17),2,4,6(19),14(18),15-hexaen-9-one |
|---|
| SMILES | COC1=CC2=CC(=C1OC)C1=C(O)C=CC(CCC(=O)CCCC2)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H24O4/c1-24-20-13-15-5-3-4-6-16(22)9-7-14-8-10-19(23)17(11-14)18(12-15)21(20)25-2/h8,10-13,23H,3-7,9H2,1-2H3 |
|---|
| InChI Key | UCIYWYZLILBGOL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as meta,meta-bridged biphenyls. These are cyclic diarylheptanoids where the two aryl groups are linked to each other by an ether group conjugated to their 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Diarylheptanoids |
|---|
| Sub Class | Cyclic diarylheptanoids |
|---|
| Direct Parent | Meta,meta-bridged biphenyls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta,meta-bridged biphenyl
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Cyclic ketone
- Ketone
- Ether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ot-0079000000-4900091edd2e912c57ec | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006t-2009000000-898c8c1c10d610cad58a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-913748d90cf816c0038e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-2179000000-933170d4386e995b258e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0292-2392000000-bb9bf2ec202f22633980 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-7ab2f31513526cd86d4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-43c6f54a933c89a959f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-6094000000-b85d7be453cd6f202686 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-7b9c90db07ee316cee16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0029000000-3ac8c53be5af70773799 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0091000000-716f6a13b1ead4f8537d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0009000000-b245455e2e6efd9c15fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-0009000000-25c29361084cc324f2ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a74-0094000000-3a666db3ed043b690673 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030799 |
|---|
| FooDB ID | FDB002740 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10273166 |
|---|
| ChEBI ID | 172562 |
|---|
| PubChem Compound ID | 21637794 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|