| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:54:20 UTC |
|---|
| Update Date | 2016-11-09 01:17:57 UTC |
|---|
| Accession Number | CHEM024754 |
|---|
| Identification |
|---|
| Common Name | Heliocide H3 |
|---|
| Class | Small Molecule |
|---|
| Description | Heliocide H3 is found in fats and oils. Heliocide H3 is a constituent of Gossypium hirsutum (cotton) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
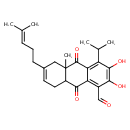
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5,8,8a,9,10,10a-Hexahydro-2,3-dihydroxy-10a-methyl-4-(1-methylethyl)-6-(4-methyl-3-pentenyl)-9,10-dioxo-1-anthracenecarboxaldehyde, 9ci | HMDB |
|
|---|
| Chemical Formula | C25H30O5 |
|---|
| Average Molecular Mass | 410.503 g/mol |
|---|
| Monoisotopic Mass | 410.209 g/mol |
|---|
| CAS Registry Number | 64960-69-6 |
|---|
| IUPAC Name | 2,3-dihydroxy-10a-methyl-6-(4-methylpent-3-en-1-yl)-9,10-dioxo-4-(propan-2-yl)-5,8,8a,9,10,10a-hexahydroanthracene-1-carbaldehyde |
|---|
| Traditional Name | 2,3-dihydroxy-4-isopropyl-10a-methyl-6-(4-methylpent-3-en-1-yl)-9,10-dioxo-8,8a-dihydro-5H-anthracene-1-carbaldehyde |
|---|
| SMILES | CC(C)C1=C2C(=O)C3(C)CC(CCC=C(C)C)=CCC3C(=O)C2=C(C=O)C(O)=C1O |
|---|
| InChI Identifier | InChI=1S/C25H30O5/c1-13(2)7-6-8-15-9-10-17-22(28)19-16(12-26)21(27)23(29)18(14(3)4)20(19)24(30)25(17,5)11-15/h7,9,12,14,17,27,29H,6,8,10-11H2,1-5H3 |
|---|
| InChI Key | YCZAHJSVHOSXPX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyanthraquinones. Hydroxyanthraquinones are compounds containing a hydroxyanthraquinone moiety, which consists of an anthracene bearing a quinone, and hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthraquinones |
|---|
| Direct Parent | Hydroxyanthraquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyanthraquinone
- Cadinane sesquiterpenoid
- Sesquiterpenoid
- Tetralin
- Quinone
- Aryl alkyl ketone
- Aryl ketone
- Aryl-aldehyde
- Vinylogous acid
- Ketone
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Aldehyde
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-1219000000-a5bc76819d4d2d6c82db | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000l-4110490000-fea0a02ed9d87b00090c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0109700000-0208160df65260dc97d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1398100000-904ceeb8be1c47e9e14c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdi-9317000000-058213fd206eafcd4511 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0001900000-e854540f12704d593c01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0115900000-5128f32acc643e244311 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-7159000000-21437f7808399497a7ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-db39b11405f8785914dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0005900000-e4fbeb6d8c2b034956bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08i0-0019100000-f3a6cc73d03668356bf8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-04229c9edae78a0f95c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0019000000-be5c7224017e744e2f9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-3249000000-98f99448cc4552f25ec8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030741 |
|---|
| FooDB ID | FDB002669 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 478980 |
|---|
| ChEBI ID | 172650 |
|---|
| PubChem Compound ID | 550597 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|