| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:53:58 UTC |
|---|
| Update Date | 2016-11-09 01:17:57 UTC |
|---|
| Accession Number | CHEM024747 |
|---|
| Identification |
|---|
| Common Name | Aurantiumal |
|---|
| Class | Small Molecule |
|---|
| Description | Aurantiumal is found in citrus. Aurantiumal is isolated from oil of grapefruit peel (Citrus paradisi |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
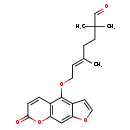
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-[(3,6-Dimethyl-6-formyl-2-heptenyl)oxy]psoralen | HMDB |
|
|---|
| Chemical Formula | C21H22O5 |
|---|
| Average Molecular Mass | 354.396 g/mol |
|---|
| Monoisotopic Mass | 354.147 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (5E)-2,2,5-trimethyl-7-({7-oxo-7H-furo[3,2-g]chromen-4-yl}oxy)hept-5-enal |
|---|
| Traditional Name | (5E)-2,2,5-trimethyl-7-({7-oxofuro[3,2-g]chromen-4-yl}oxy)hept-5-enal |
|---|
| SMILES | C\C(CCC(C)(C)C=O)=C/COC1=C2C=COC2=CC2=C1C=CC(=O)O2 |
|---|
| InChI Identifier | InChI=1S/C21H22O5/c1-14(6-9-21(2,3)13-22)7-10-25-20-15-4-5-19(23)26-18(15)12-17-16(20)8-11-24-17/h4-5,7-8,11-13H,6,9-10H2,1-3H3/b14-7+ |
|---|
| InChI Key | JBBNVKWFZYFPIG-VGOFMYFVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as psoralens. These are organic compounds containing a psoralen moiety, which consists of a furan fused to a chromenone to for 7H-furo[3,2-g]chromen-7-one. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | Psoralens |
|---|
| Alternative Parents | |
|---|
| Substituents | - Psoralen
- Benzopyran
- 1-benzopyran
- Benzofuran
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Pyran
- Heteroaromatic compound
- Furan
- Lactone
- Ether
- Oxacycle
- Organoheterocyclic compound
- Aldehyde
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fi0-4569000000-18a7a96accf2ad0d4300 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0329000000-3ba30c824120dddd5cb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-5795000000-f44ed3b5b7097fd2e440 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9640000000-f522d76819173300aed8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0039000000-7a12ac609e967bb30c8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0494000000-58d184a896d50bc7e900 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1910000000-f16468f2722290f6f4ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0198000000-7da23b8a6046a154588b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-3696000000-d0729de9efec49c7b2cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kxr-3972000000-96957b4a02c2059fe29d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fb9-0059000000-240036c4fb670a837be4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0398000000-7db2b7ffa0d354d734ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zfr-0890000000-d66a954f8c5efe2e2fdd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030735 |
|---|
| FooDB ID | FDB002661 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776846 |
|---|
| ChEBI ID | 171795 |
|---|
| PubChem Compound ID | 122201415 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|