| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:53:14 UTC |
|---|
| Update Date | 2016-11-09 01:17:56 UTC |
|---|
| Accession Number | CHEM024728 |
|---|
| Identification |
|---|
| Common Name | (R)-3',7-Dihydroxy-2',4'-dimethoxyisoflavan |
|---|
| Class | Small Molecule |
|---|
| Description | (±)-3',7-Dihydroxy-2',4'-dimethoxyisoflavan is found in common bean. (±)-3',7-Dihydroxy-2',4'-dimethoxyisoflavan is isolated from Astragalus gummifer (tragacanth |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
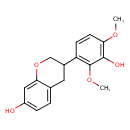
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+/-)-mucronulatol | ChEMBL, HMDB | | (+)-Mucronulatol | HMDB | | (R)-Mucronulatol | HMDB | | 3',7-Dihydroxy-2',4'-dimethoxyisoflavan | MeSH, HMDB | | Mucronulatol | MeSH |
|
|---|
| Chemical Formula | C17H18O5 |
|---|
| Average Molecular Mass | 302.322 g/mol |
|---|
| Monoisotopic Mass | 302.115 g/mol |
|---|
| CAS Registry Number | 57128-11-7 |
|---|
| IUPAC Name | 3-(3-hydroxy-2,4-dimethoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| Traditional Name | 3-(3-hydroxy-2,4-dimethoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| SMILES | COC1=C(O)C(OC)=C(C=C1)C1COC2=CC(O)=CC=C2C1 |
|---|
| InChI Identifier | InChI=1S/C17H18O5/c1-20-14-6-5-13(17(21-2)16(14)19)11-7-10-3-4-12(18)8-15(10)22-9-11/h3-6,8,11,18-19H,7,9H2,1-2H3 |
|---|
| InChI Key | NUNFZNIXYWTZMW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3'-hydroxy,4'-methoxyisoflavonoids. These are isoflavonoids carrying a methoxy group attached to the C4' atom, as well as a hydroxyl group at the C3'-position of the isoflavonoid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | O-methylated isoflavonoids |
|---|
| Direct Parent | 3'-hydroxy,4'-methoxyisoflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3'-hydroxy,4'-methoxyisoflavonoid
- 2p-methoxyisoflavonoid-skeleton
- Isoflavanol
- Hydroxyisoflavonoid
- Isoflavan
- Benzopyran
- M-dimethoxybenzene
- Dimethoxybenzene
- Methoxyphenol
- Chromane
- 1-benzopyran
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- Oxacycle
- Organoheterocyclic compound
- Ether
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-0792000000-45287359a30832a00bf4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0089-3223900000-1d8c1817a182ade16a80 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0918000000-8fff6673a2556321d6ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0951000000-a5b30f6265793cc88044 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-1910000000-0a6f205c78f9fb160c3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0319000000-9124c07fc707d1cac393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0974000000-a61ab342c30210b24c47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-2930000000-5cffb04e464eaa08989a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udj-0918000000-2fdcaa21ae60fc2090a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6t-0911000000-403150e58ae5ea81bb1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00yi-0910000000-3fbd93eeb7de3c410e4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-c1d1ac47665b26293f56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ukj-0392000000-ad40b960e43bcabf51b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-1790000000-d1c0755a72db13dbb5bf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030717 |
|---|
| FooDB ID | FDB002640 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00009715 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 3682776 |
|---|
| ChEBI ID | 605421 |
|---|
| PubChem Compound ID | 4484949 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|