| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:52:55 UTC |
|---|
| Update Date | 2016-11-09 01:17:56 UTC |
|---|
| Accession Number | CHEM024722 |
|---|
| Identification |
|---|
| Common Name | Cyclomammein |
|---|
| Class | Small Molecule |
|---|
| Description | Cyclomammein is found in fruits. Cyclomammein is found in seeds of Mammea americana (mamey) Beta-D-Glucose 6 phosphate (b-G6P) is the beta-anomer of glucose-6-phosphate. There are two anomers of glucose 6 phosphate, the alpha anomer and the beta anomer. Specifically, beta-D-Glucose 6-phosphate is glucose sugar phosphorylated on carbon 6. It is a very common metabolite in cells as the vast majority of glucose entering a cell will become phosphorylated in this way. The primary reason for the immediate phosphorylation of glucose is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane. b-G6P is involved in the glycolysis, gluconeogenesis, pentose phosphate, and glycogen and sucrose metabolic pathways [Kegg ID: C01172]. Beta-D-Glucose 6 phosphate can be generated through beta-D-fructose phosphate or alpha-D-glucose 6 phosphate (via glucose-6-phosphate isomerase) or beta-D glucose (via hexokinase). It can then be sent off to the pentose phosphate pathway which generates the useful cofactor NADPH as well as ribulose 5-phosphate, a carbon source for the synthesis of other molecules. Alternately if the cell needs energy or carbon skeletons for synthesis then glucose 6-phosphate is targeted for glycolysis. A third route is to have glucose 6 phosphate stored or converted to glycogen, especially if blood glucose levels are high |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
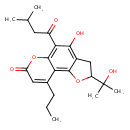
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Dihydro-4-hydroxy-2-(1-hydroxy-1-methylethyl)-5-(3-methyl-1-oxobutyl)-9-propyl-7H-furo[2,3-F][1]benzopyran-7-one, 9ci | HMDB | | Mammea b/ba cyclo F | HMDB |
|
|---|
| Chemical Formula | C22H28O6 |
|---|
| Average Molecular Mass | 388.454 g/mol |
|---|
| Monoisotopic Mass | 388.189 g/mol |
|---|
| CAS Registry Number | 30390-03-5 |
|---|
| IUPAC Name | 4-hydroxy-2-(2-hydroxypropan-2-yl)-5-(3-methylbutanoyl)-9-propyl-2H,3H,7H-furo[2,3-f]chromen-7-one |
|---|
| Traditional Name | 4-hydroxy-2-(2-hydroxypropan-2-yl)-5-(3-methylbutanoyl)-9-propyl-2H,3H-furo[2,3-f]chromen-7-one |
|---|
| SMILES | CCCC1=CC(=O)OC2=C(C(=O)CC(C)C)C(O)=C3CC(OC3=C12)C(C)(C)O |
|---|
| InChI Identifier | InChI=1S/C22H28O6/c1-6-7-12-9-16(24)28-21-17(12)20-13(10-15(27-20)22(4,5)26)19(25)18(21)14(23)8-11(2)3/h9,11,15,25-26H,6-8,10H2,1-5H3 |
|---|
| InChI Key | TVYLJAPSHFFCMT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as angular furanocoumarins. These are furanocoumarins, with a structure characterized by a furan ring angularly fused to a coumarin. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | Angular furanocoumarins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Angular furanocoumarin
- Butyrophenone
- Benzopyran
- 1-benzopyran
- Coumaran
- Aryl alkyl ketone
- Aryl ketone
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Pyran
- Heteroaromatic compound
- Vinylogous acid
- Tertiary alcohol
- Ketone
- Lactone
- Oxacycle
- Ether
- Organoheterocyclic compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9117000000-5d493af962d2fd183f9c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00lr-8930460000-d3147a33d266fa3da4f7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-0009000000-e41e8cc95a14ef361504 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08ni-3009000000-05b5963ff0f2f1d22a3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-3090000000-8128462a77a70729e3f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-bd86472b6b59efaaa5f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f79-3039000000-bf48a7c8c091389e8aa3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-5193000000-fa8b3c727ecc88503e60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-7f16ac45973a6479b3e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-371214e0574db81e9c8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gbl-4049000000-2dca35d98f281d3c9f3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-9e42c3387fe4f6435022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0009000000-f0bb3a2d86584713a762 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfs-4189000000-4d65cade4de35f951c91 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030711 |
|---|
| FooDB ID | FDB002633 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054441 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013256 |
|---|
| ChEBI ID | 175940 |
|---|
| PubChem Compound ID | 101967071 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|