| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:51:53 UTC |
|---|
| Update Date | 2016-11-09 01:17:56 UTC |
|---|
| Accession Number | CHEM024693 |
|---|
| Identification |
|---|
| Common Name | Diplosporin |
|---|
| Class | Small Molecule |
|---|
| Description | Epi-diplosporin is a metabolite of Diplodia macrospora isolated from infected maize. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
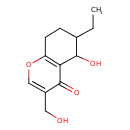
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5S,6X)-6-Ethyl-5-hydroxy-3-hydroxymethyl-5,6,7,8-tetrahydrobenzo[b]pyran-4-one | HMDB | | 6-Ethyl-5,6,7,8-tetrahydro-5-hydroxy-3-(hydroxymethyl)-4H-1-benzopyran-4-one, 9ci | HMDB | | Diplodiol | HMDB | | trans-6-Ethyl-5-hydroxy-3-hydroxymethyl-5,6,7,8-tetrahydrochromone | HMDB |
|
|---|
| Chemical Formula | C12H16O4 |
|---|
| Average Molecular Mass | 224.253 g/mol |
|---|
| Monoisotopic Mass | 224.105 g/mol |
|---|
| CAS Registry Number | 69199-05-9 |
|---|
| IUPAC Name | 6-ethyl-5-hydroxy-3-(hydroxymethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one |
|---|
| Traditional Name | 6-ethyl-5-hydroxy-3-(hydroxymethyl)-5,6,7,8-tetrahydrochromen-4-one |
|---|
| SMILES | CCC1CCC2=C(C1O)C(=O)C(CO)=CO2 |
|---|
| InChI Identifier | InChI=1S/C12H16O4/c1-2-7-3-4-9-10(11(7)14)12(15)8(5-13)6-16-9/h6-7,11,13-14H,2-5H2,1H3 |
|---|
| InChI Key | BFQQKLXDPGTJKC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranones and derivatives. Pyranones and derivatives are compounds containing a pyran ring which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrans |
|---|
| Sub Class | Pyranones and derivatives |
|---|
| Direct Parent | Pyranones and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranone
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic alcohol
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-054p-3930000000-8c6a2beae4a48b4b64d1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fi0-8479000000-aa0c870bc08d54ae44c2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0390000000-1bb1de37c70bccd57e21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-5960000000-764866caa441e7f1d68e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zg0-9800000000-81ce6fc4e7efb61d1696 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0290000000-be1e6669da5e485eb6f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-0920000000-d4f31c7ed71b1f49cbb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fyt-4900000000-5a63c2ef53e887a5bdfe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-2cda259b7fc3f9ccffb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1590000000-88dbfafa523c665e4122 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fs-9810000000-c24542c68e27e1116fe9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-ab8983f3efe4ba8e9388 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1690000000-424b32db727753d66f4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0avi-6910000000-59071c93c73564ef4f6e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030680 |
|---|
| FooDB ID | FDB020101 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024012 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 28583680 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14208867 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|