| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:50:33 UTC |
|---|
| Update Date | 2016-11-09 01:17:56 UTC |
|---|
| Accession Number | CHEM024657 |
|---|
| Identification |
|---|
| Common Name | delta-Amorphene |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the cadinene family of sesquiterpenes in which the double bonds are located at the 4-4a and 7-8 positions, and in which the isopropyl group at position 1 is cis to the hydrogen at the adjacent bridgehead carbon (position 8a). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
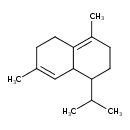
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,7-Dimethyl-1-(propan-2-yl)-1,2,3,5,6,8a-hexahydronaphthalene | ChEBI | | 4,7-Dimethyl-1-isopropyl-1,2,3,5,6,8a-hexahydronaphthalene | ChEBI | | Δ-amorphene | Generator | | (+)-delta-Amorphene | HMDB | | D-Amorphene | HMDB | | Δ-cadinene | Generator |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | 189165-79-5 |
|---|
| IUPAC Name | 4,7-dimethyl-1-(propan-2-yl)-1,2,3,5,6,8a-hexahydronaphthalene |
|---|
| Traditional Name | 4-isopropyl-1,6-dimethyl-2,3,4,4a,7,8-hexahydronaphthalene |
|---|
| SMILES | CC(C)C1CCC(C)=C2CCC(C)=CC12 |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-10(2)13-8-6-12(4)14-7-5-11(3)9-15(13)14/h9-10,13,15H,5-8H2,1-4H3 |
|---|
| InChI Key | FUCYIEXQVQJBKY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Cadinane sesquiterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p5-2900000000-1c78db8ac8cea1f18f19 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0490000000-85ec97b8032d4370cfe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bta-2920000000-51a0b00ff6967440ef07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02bk-5900000000-d0bfeebca8e74869b58e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-fe8dc7c5f863461e974f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-55a43b599b1e9bc5b16a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0h2s-0910000000-6ad78804a6c28a720abf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pvv-0920000000-b1bdc39be78c614df14d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0390000000-9cc73bda60352fda947a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4s-5940000000-9954c90e487d5cf37532 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-785f0b3268eb469c47c1 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-08gl-5910000000-9a4d0fed838cbfb4f726 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030644 |
|---|
| FooDB ID | FDB021731 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00037022 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9806 |
|---|
| ChEBI ID | 140564 |
|---|
| PubChem Compound ID | 10223 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|