| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:47:24 UTC |
|---|
| Update Date | 2016-11-09 01:17:55 UTC |
|---|
| Accession Number | CHEM024569 |
|---|
| Identification |
|---|
| Common Name | Trisjuglone |
|---|
| Class | Small Molecule |
|---|
| Description | Trisjuglone is found in common walnut. Trisjuglone is a constituent of Juglans regia (walnut) roots |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
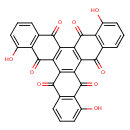
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,7,13-Trihydroxy-5,6,11,12,17,18-trinaphthylenehexone, 9ci | HMDB | | Cyclotrijuglone | HMDB |
|
|---|
| Chemical Formula | C30H12O9 |
|---|
| Average Molecular Mass | 516.411 g/mol |
|---|
| Monoisotopic Mass | 516.048 g/mol |
|---|
| CAS Registry Number | 50838-55-6 |
|---|
| IUPAC Name | 5,15,25-trihydroxyheptacyclo[20.8.0.0²,¹¹.0⁴,⁹.0¹²,²¹.0¹⁴,¹⁹.0²⁴,²⁹]triaconta-1(22),2(11),4(9),5,7,12(21),14(19),15,17,24(29),25,27-dodecaene-3,10,13,20,23,30-hexone |
|---|
| Traditional Name | 5,15,25-trihydroxyheptacyclo[20.8.0.0²,¹¹.0⁴,⁹.0¹²,²¹.0¹⁴,¹⁹.0²⁴,²⁹]triaconta-1(22),2(11),4(9),5,7,12(21),14(19),15,17,24(29),25,27-dodecaene-3,10,13,20,23,30-hexone |
|---|
| SMILES | OC1=CC=CC2=C1C(=O)C1=C(C2=O)C2=C(C(=O)C3=C(C(O)=CC=C3)C2=O)C2=C1C(=O)C1=C(C(O)=CC=C1)C2=O |
|---|
| InChI Identifier | InChI=1S/C30H12O9/c31-13-7-1-4-10-16(13)28(37)22-19(25(10)34)23-21(27(36)11-5-2-8-14(32)17(11)29(23)38)24-20(22)26(35)12-6-3-9-15(33)18(12)30(24)39/h1-9,31-33H |
|---|
| InChI Key | DNGCQXLPUZWYNB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triphenylenes. Triphenylenes are compounds containing a triphenylene moiety, which consists of four fused benzene rings forming a 9,10-benzo[l]phenanthrene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenanthrenes and derivatives |
|---|
| Sub Class | Triphenylenes |
|---|
| Direct Parent | Triphenylenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triphenylene
- Anthracene
- 1-naphthol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Vinylogous acid
- Polyol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01b9-0404590000-d3302e52b53475c5e045 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00yi-4300449000-e01c71e073cf399c897e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000090000-526cc6eac3eb9e98a919 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000090000-c64667e54785afad683c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-3000390000-397a06e51c53aaa62688 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-1de4d8068926c2c256de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000090000-1de4d8068926c2c256de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3000190000-87782640ee209bb7ada6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-db4b31645b06bb0a2e63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000090000-db4b31645b06bb0a2e63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-0000900000-a408c51af00a7657b537 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000090000-1f26c9555551f8f164f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000090000-1f26c9555551f8f164f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-2409810000-721ad39ca6abb1374eaf | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030568 |
|---|
| FooDB ID | FDB002454 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001102 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776836 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 90473021 |
|---|
| Kegg Compound ID | C10187 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|