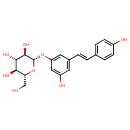
| 3,4',5-Trihydroxystilbene-3-beta-D-glucoside | ChEBI |
| 3,4,5-Trihydroxystilbene-3-beta-monoglucoside | ChEBI |
| Piceid | ChEBI |
| Polydatin | ChEBI |
| trans-Resveratrol 3-beta-D-glucoside | ChEBI |
| trans-Resveratrol 3-beta-glucoside | ChEBI |
| trans-Resveratrol 3-O-beta-D-glucoside | ChEBI |
| 3,4',5-Trihydroxystilbene-3-b-D-glucoside | Generator |
| 3,4',5-Trihydroxystilbene-3-β-D-glucoside | Generator |
| 3,4,5-Trihydroxystilbene-3-b-monoglucoside | Generator |
| 3,4,5-Trihydroxystilbene-3-β-monoglucoside | Generator |
| trans-Resveratrol 3-b-D-glucoside | Generator |
| trans-Resveratrol 3-β-D-glucoside | Generator |
| trans-Resveratrol 3-b-glucoside | Generator |
| trans-Resveratrol 3-β-glucoside | Generator |
| trans-Resveratrol 3-O-b-D-glucoside | Generator |
| trans-Resveratrol 3-O-β-D-glucoside | Generator |
| 3,4'-5-Trihydroxystilbene-3-beta-D-glucopyranoside | HMDB |
| 3,5,4'-Trihydroxystilbene 3-glucoside | HMDB |
| Resveratrol 3-beta-D-glucoside | HMDB |
| Resveratrol 3-beta-mono-D-glucoside | HMDB |
| Resveratrol 3-glucoside | HMDB |
| Resveratrol 3-O-glucoside | HMDB |
| Resveratrol 5-O-glucoside | HMDB |
| trans-Resveratrol 3-glucoside | HMDB |
| trans-Resveratrol 3-O-glucoside | HMDB |
| 3,4,5-TSG | MeSH |
| Polydatin, (e)-isomer | MeSH |
| Resveratrol 3-O-beta-D-glucoside | MeSH |
| 3-Hydroxy-5-(2-(4-hydroxyphenyl)ethenyl)phenyl-beta-D-glucoside | MeSH |
| 5,4'-Dihydroxystilbene-3-O-beta-D-GLCP | MeSH |
| 3-Hydroxy-5-[(1E)-2-(4-hydroxyphenyl)ethenyl]phenyl beta-D-glucopyranoside | PhytoBank |
| 3-Hydroxy-5-[(1E)-2-(4-hydroxyphenyl)ethenyl]phenyl β-D-glucopyranoside | PhytoBank |
| (-)-trans-Resveratrol 13-O-beta-D-glucopyranoside | PhytoBank |
| (-)-trans-Resveratrol 13-O-β-D-glucopyranoside | PhytoBank |
| (E)-Piceid | PhytoBank |
| (E)-Polydatin | PhytoBank |
| (E)-Resveratrol 3-O-beta-D-glucopyranoside | PhytoBank |
| (E)-Resveratrol 3-O-β-D-glucopyranoside | PhytoBank |
| 3-Hydroxy-5-(p-hydroxystyryl)phenyl beta-D-glucoside | PhytoBank |
| 3-Hydroxy-5-(p-hydroxystyryl)phenyl β-D-glucoside | PhytoBank |
| 3-O-Glucosyl-resveratrol | PhytoBank |
| Pieceid | PhytoBank |
| Polydotin peceid | PhytoBank |
| Resveratrol 3-O-β-D-glucoside | PhytoBank |
| Resveratrol 3-O-beta-glucopyranoside | PhytoBank |
| Resveratrol 3-O-β-glucopyranoside | PhytoBank |
| Resveratrol beta-D-glucoside | PhytoBank |
| Resveratrol β-D-glucoside | PhytoBank |
| Resveratrol beta-glucoside | PhytoBank |
| Resveratrol β-glucoside | PhytoBank |
| Resveratrol glucoside | PhytoBank |
| trans-Piceid | PhytoBank |
| trans-Polydatin | PhytoBank |
| trans-3,5,4'-Trihydroxystilbene 3-O-beta-glucopyranoside | PhytoBank |
| trans-3,5,4'-Trihydroxystilbene 3-O-β-glucopyranoside | PhytoBank |
| trans-3,5,4’-Trihydroxystilbene 3-O-β-glucopyranoside | PhytoBank |
| trans-3,5,4'-Trihydroxystilbene 3-O-beta-glucoside | PhytoBank |
| trans-3,5,4'-Trihydroxystilbene 3-O-β-glucoside | PhytoBank |
| trans-3,5,4’-Trihydroxystilbene 3-O-β-glucoside | PhytoBank |
| trans-3,5,4'-Trihydroxystilbene 3-O-glucoside | PhytoBank |
| trans-3,5,4’-Trihydroxystilbene 3-O-glucoside | PhytoBank |
| trans-3,5,4'-Trihydroxystilbene 3-glucoside | PhytoBank |
| trans-3,5,4’-Trihydroxystilbene 3-glucoside | PhytoBank |
| 3,5,4'-Trihydroxystilbene 3-O-beta-glucopyranoside | PhytoBank |
| 3,5,4'-Trihydroxystilbene 3-O-β-glucopyranoside | PhytoBank |
| 3,5,4’-Trihydroxystilbene 3-O-β-glucopyranoside | PhytoBank |
| 3,5,4'-Trihydroxystilbene 3-O-beta-glucoside | PhytoBank |
| 3,5,4'-Trihydroxystilbene 3-O-β-glucoside | PhytoBank |
| 3,5,4’-Trihydroxystilbene 3-O-β-glucoside | PhytoBank |
| 3,5,4'-Trihydroxystilbene 3-O-glucoside | PhytoBank |
| 3,5,4’-Trihydroxystilbene 3-O-glucoside | PhytoBank |
| 3,5,4’-Trihydroxystilbene 3-glucoside | PhytoBank |