| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:47:02 UTC |
|---|
| Update Date | 2016-11-09 01:17:55 UTC |
|---|
| Accession Number | CHEM024560 |
|---|
| Identification |
|---|
| Common Name | m-Trigallic acid |
|---|
| Class | Small Molecule |
|---|
| Description | m-Trigallic acid is found in fruits. m-Trigallic acid is a constituent of mango (Mangifera indica) fruit |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
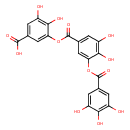
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| m-Trigallate | Generator | | 3,4-Dihydroxy-5-[(3,4,5-trihydroxybenzoyl)oxy]benzoic acid 5-carboxy-2,3-dihydroxyphenyl ester, 9ci | HMDB | | Metatrigallic acid | HMDB | | 3-[3,4-Dihydroxy-5-(3,4,5-trihydroxybenzoyloxy)benzoyloxy]-4,5-dihydroxybenzoate | HMDB |
|
|---|
| Chemical Formula | C21H14O13 |
|---|
| Average Molecular Mass | 474.328 g/mol |
|---|
| Monoisotopic Mass | 474.043 g/mol |
|---|
| CAS Registry Number | 2131-66-0 |
|---|
| IUPAC Name | 3-[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyloxy)benzoyloxy]-4,5-dihydroxybenzoic acid |
|---|
| Traditional Name | 3-[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyloxy)benzoyloxy]-4,5-dihydroxybenzoic acid |
|---|
| SMILES | OC(=O)C1=CC(O)=C(O)C(OC(=O)C2=CC(O)=C(O)C(OC(=O)C3=CC(O)=C(O)C(O)=C3)=C2)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H14O13/c22-10-2-8(3-11(23)16(10)26)20(31)34-15-6-9(4-13(25)18(15)28)21(32)33-14-5-7(19(29)30)1-12(24)17(14)27/h1-6,22-28H,(H,29,30) |
|---|
| InChI Key | QUXNYZHQBWMPNX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as depsides and depsidones. These are polycyclic compounds that is either a polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond (depside), or a compound containing the depsidone structure (depsidone). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Depsides and depsidones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Depsides and depsidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Depside backbone
- Galloyl ester
- Gallic acid or derivatives
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Dihydroxybenzoic acid
- Hydroxybenzoic acid
- Phenol ester
- Benzoate ester
- Benzoic acid
- Benzenetriol
- Benzoic acid or derivatives
- Pyrogallol derivative
- Tricarboxylic acid or derivatives
- Benzoyl
- Catechol
- Phenoxy compound
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Carboxylic acid ester
- Polyol
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-0911000000-348d08e7df842a98eddc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0fb9-1907002000-e4ece7d758ae94f7e088 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kdi-0614900000-1201f6bb101c9c3994ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zi0-0923400000-b206f66b6cd63de0a5e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-1920000000-5857397eaa90ceb95041 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-0301900000-152f4b8c99231f10c7b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fi0-0922600000-2b4631ad14bcd50bdefa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gdi-0900000000-78388e036ee73fdef5ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0402900000-221aeaad772eaf427bbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0304900000-b460ee4d5fe540b5636e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-4901400000-50fb4771036911b3007e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0300900000-19a355d3bb50327d98cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0m90-0912600000-af8bdfaf5d9cc5384cae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-3690600000-699040b3623e3a60d23f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030559 |
|---|
| FooDB ID | FDB002443 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00058017 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776834 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 90470472 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|