| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:45:41 UTC |
|---|
| Update Date | 2016-11-09 01:17:54 UTC |
|---|
| Accession Number | CHEM024523 |
|---|
| Identification |
|---|
| Common Name | Citreoviridin D |
|---|
| Class | Small Molecule |
|---|
| Description | Citreoviridin D is a mycotoxin produced by the common soil organism Aspergillus terreu |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C24H32O6 |
|---|
| Average Molecular Mass | 416.507 g/mol |
|---|
| Monoisotopic Mass | 416.220 g/mol |
|---|
| CAS Registry Number | 74145-79-2 |
|---|
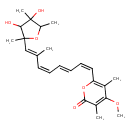
| IUPAC Name | 6-[(1Z,3E,5Z,7E)-8-(3,4-dihydroxy-2,4,5-trimethyloxolan-2-yl)-7-methylocta-1,3,5,7-tetraen-1-yl]-4-methoxy-3,5-dimethyl-2H-pyran-2-one |
|---|
| Traditional Name | 6-[(1Z,3E,5Z,7E)-8-(3,4-dihydroxy-2,4,5-trimethyloxolan-2-yl)-7-methylocta-1,3,5,7-tetraen-1-yl]-4-methoxy-3,5-dimethylpyran-2-one |
|---|
| SMILES | COC1=C(C)C(=O)OC(\C=C/C=C/C=C\C(\C)=C\C2(C)OC(C)C(C)(O)C2O)=C1C |
|---|
| InChI Identifier | InChI=1S/C24H32O6/c1-15(14-23(5)22(26)24(6,27)18(4)30-23)12-10-8-9-11-13-19-16(2)20(28-7)17(3)21(25)29-19/h8-14,18,22,26-27H,1-7H3/b9-8+,12-10-,13-11-,15-14+ |
|---|
| InChI Key | WQOZGNFAVRFSGE-ULPXYLRASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranones and derivatives. Pyranones and derivatives are compounds containing a pyran ring which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrans |
|---|
| Sub Class | Pyranones and derivatives |
|---|
| Direct Parent | Pyranones and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl aryl ether
- Pyranone
- Heteroaromatic compound
- Vinylogous ester
- Tetrahydrofuran
- Tertiary alcohol
- 1,2-diol
- Lactone
- Secondary alcohol
- Dialkyl ether
- Ether
- Oxacycle
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9015100000-003c9f853a77437736d7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-002k-7333190000-a6454fb1dd3860c510bd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0388900000-1552bb733536ce8260bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06dj-0492100000-9f76595e150b2f1c7adb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbc-9630000000-78d4c34d00bcb269ac3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-2106900000-a4e209b92464d6e80b81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066s-5309300000-a199cd6396d5be2ec6c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0604-9438000000-36e836e0414183dde9bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0397400000-9268876f7257705638bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0692000000-0753b29a85633ae6b69d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1000-1950000000-3ca1cc7b41acd18b678f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0102900000-594d94b8103a47844a75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0frt-0039100000-e3860c6793208f7f6e70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-6394000000-52107a1a9f54cdaa674a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030522 |
|---|
| FooDB ID | FDB002394 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055195 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013220 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751043 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|