| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:45:37 UTC |
|---|
| Update Date | 2016-11-09 01:17:54 UTC |
|---|
| Accession Number | CHEM024521 |
|---|
| Identification |
|---|
| Common Name | Virginiamycin |
|---|
| Class | Small Molecule |
|---|
| Description | Antibacterial food additive. Discontinued Virginiamycin is a streptogramin antibiotic similar to pristinamycin and quinupristin/dalfopristin. It is a combination of pristinamycin IIA (virginiamycin M1) and virginiamycin S1. Virginiamycin is used in the fuel ethanol industry to prevent microbial contamination |
|---|
| Contaminant Sources | - FooDB Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
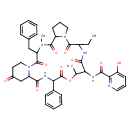
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Virginiamycin factor S | Kegg | | Antibiotic 1754Z3b | HMDB | | Cebin V | HMDB | | Eskalin V | HMDB | | Eskamicin | HMDB | | Mikamycin | HMDB | | Ostreogrycin | HMDB | | Patricin | HMDB | | Pristinamycin | HMDB | | Stafac | HMDB | | Staphylomycin S | HMDB | | Staphylomycin S1 | HMDB | | Stephylomycin | HMDB | | Streptogramin | HMDB | | Vernamycin | HMDB | | Virginiamycin factor S1 | HMDB | | Virginiamycin S1 | HMDB | | Virginiamycin, ban, inn, usan | HMDB | | VirginiamycinVirginiamycin S1 | HMDB | | Dihydrovirginiamycin S1 | HMDB |
|
|---|
| Chemical Formula | C43H49N7O10 |
|---|
| Average Molecular Mass | 823.890 g/mol |
|---|
| Monoisotopic Mass | 823.354 g/mol |
|---|
| CAS Registry Number | 11006-76-1 |
|---|
| IUPAC Name | N-{3-benzyl-12-ethyl-4,16-dimethyl-2,5,11,14,18,21,24-heptaoxo-19-phenyl-17-oxa-1,4,10,13,20-pentaazatricyclo[20.4.0.0⁶,¹⁰]hexacosan-15-yl}-3-hydroxypyridine-2-carboxamide |
|---|
| Traditional Name | virginiamycin factor S1 |
|---|
| SMILES | CCC1NC(=O)C(NC(=O)C2=NC=CC=C2O)C(C)OC(=O)C(NC(=O)C2CC(=O)CCN2C(=O)C(CC2=CC=CC=C2)N(C)C(=O)C2CCCN2C1=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C43H49N7O10/c1-4-29-40(56)49-21-12-17-30(49)41(57)48(3)32(23-26-13-7-5-8-14-26)42(58)50-22-19-28(51)24-31(50)37(53)47-35(27-15-9-6-10-16-27)43(59)60-25(2)34(38(54)45-29)46-39(55)36-33(52)18-11-20-44-36/h5-11,13-16,18,20,25,29-32,34-35,52H,4,12,17,19,21-24H2,1-3H3,(H,45,54)(H,46,55)(H,47,53) |
|---|
| InChI Key | FEPMHVLSLDOMQC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclic depsipeptides. These are natural or synthetic compounds having sequences of amino and hydroxy carboxylic acid residues (usually α-amino and α-hydroxy acids) connected in a ring. The residues are commonly but not necessarily regularly alternating. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Peptidomimetics |
|---|
| Sub Class | Depsipeptides |
|---|
| Direct Parent | Cyclic depsipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclic depsipeptide
- Macrolide lactam
- Alpha-amino acid ester
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Pyridine carboxylic acid or derivatives
- Pyridinecarboxamide
- 2-heteroaryl carboxamide
- Hydroxypyridine
- Piperidinone
- Benzenoid
- Pyridine
- Monocyclic benzene moiety
- Piperidine
- Vinylogous acid
- Tertiary carboxylic acid amide
- Pyrrolidine
- Heteroaromatic compound
- Carboxamide group
- Carboxylic acid ester
- Ketone
- Lactam
- Lactone
- Cyclic ketone
- Secondary carboxylic acid amide
- Oxacycle
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-2491106050-93de58c83883a820420e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05ai-7921000310-b3dbe42a9b0a7d161ba5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056s-6931000000-b83f8bea2e7202206d6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-2794042000-228fdff1ed817529fbc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-7941143600-2fb816345cceb90bfd5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002r-2963230100-18b6ad489184b42dfdd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1000000290-a996fb04fb507fda738c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000001840-fc34e7a46980c6034e72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000100-ac3c127095b860829f0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0100000290-a615f7321a3bda6f5f0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-5400000490-d7eb31a64f7bc5ada5bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00p1-6000000910-3ae72f4dd6f1d934b611 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030520 |
|---|
| FooDB ID | FDB002392 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Virginiamycin |
|---|
| Chemspider ID | 109411 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 122731 |
|---|
| Kegg Compound ID | C11269 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|