| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:44:47 UTC |
|---|
| Update Date | 2016-11-09 01:17:54 UTC |
|---|
| Accession Number | CHEM024507 |
|---|
| Identification |
|---|
| Common Name | Bargustanine |
|---|
| Class | Small Molecule |
|---|
| Description | Bargustanine is found in tea. Bargustanine is an alkaloid from roots of Berberis vulgaris (barberry |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
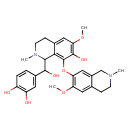
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C29H34N2O7 |
|---|
| Average Molecular Mass | 522.590 g/mol |
|---|
| Monoisotopic Mass | 522.237 g/mol |
|---|
| CAS Registry Number | 169626-12-4 |
|---|
| IUPAC Name | 4-[hydroxy({7-hydroxy-6-methoxy-8-[(6-methoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-7-yl)oxy]-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl})methyl]benzene-1,2-diol |
|---|
| Traditional Name | 4-[hydroxy({7-hydroxy-6-methoxy-8-[(6-methoxy-2-methyl-3,4-dihydro-1H-isoquinolin-7-yl)oxy]-2-methyl-3,4-dihydro-1H-isoquinolin-1-yl})methyl]benzene-1,2-diol |
|---|
| SMILES | COC1=C(OC2=C3C(C(O)C4=CC(O)=C(O)C=C4)N(C)CCC3=CC(OC)=C2O)C=C2CN(C)CCC2=C1 |
|---|
| InChI Identifier | InChI=1S/C29H34N2O7/c1-30-9-7-16-12-22(36-3)23(14-19(16)15-30)38-29-25-17(13-24(37-4)28(29)35)8-10-31(2)26(25)27(34)18-5-6-20(32)21(33)11-18/h5-6,11-14,26-27,32-35H,7-10,15H2,1-4H3 |
|---|
| InChI Key | AYINLWLMPMZNKE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzylisoquinolines. These are organic compounds containing an isoquinoline to which a benzyl group is attached. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoquinolines and derivatives |
|---|
| Sub Class | Benzylisoquinolines |
|---|
| Direct Parent | Benzylisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzylisoquinoline
- Diaryl ether
- Tetrahydroisoquinoline
- Anisole
- Catechol
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- 1,2-aminoalcohol
- Tertiary aliphatic amine
- Secondary alcohol
- Tertiary amine
- Ether
- Azacycle
- Organonitrogen compound
- Aromatic alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Alcohol
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0139000000-5c2d07311f46a0d63928 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0udi-3364829000-fef901c2539328d72676 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0100290000-54c20196f9c81b92b9fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053i-0906230000-e654910f6d5502da5d71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053i-2901000000-b46971e74d6b6bf5b610 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0102090000-93b42573724aed330839 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kou-0405290000-ddbc3bf404f83708daef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-0932000000-4db961584f1fd2a550e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0002190000-1ce597ecd8ff4f897241 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006t-0208890000-7664f46d0274f837e878 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4749310000-fcb8a3235f4701ccf150 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000090000-15bd0e23c4f830af4e2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fe0-0407910000-57ffcd2ead907984049b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2304590000-44f75d784bc2ab7b21ee | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030505 |
|---|
| FooDB ID | FDB002376 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027521 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013216 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 73813822 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|