| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:43:48 UTC |
|---|
| Update Date | 2016-11-09 01:17:54 UTC |
|---|
| Accession Number | CHEM024484 |
|---|
| Identification |
|---|
| Common Name | Aflatoxin GM2 |
|---|
| Class | Small Molecule |
|---|
| Description | Aflatoxin GM2 is a minor mycotoxin produced by Aspergillus flavus and Aspergillus parasiticus (Hugo Vanden Bossche, D.W.R. Mackenzie and G. Cauwenbergh. Aspergillus and Aspergillosis, 1987). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
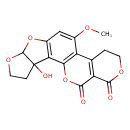
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C17H14O8 |
|---|
| Average Molecular Mass | 346.288 g/mol |
|---|
| Monoisotopic Mass | 346.069 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-hydroxy-11-methoxy-6,8,16,20-tetraoxapentacyclo[10.8.0.0²,⁹.0³,⁷.0¹³,¹⁸]icosa-1(12),2(9),10,13(18)-tetraene-17,19-dione |
|---|
| Traditional Name | 3-hydroxy-11-methoxy-6,8,16,20-tetraoxapentacyclo[10.8.0.0²,⁹.0³,⁷.0¹³,¹⁸]icosa-1(12),2(9),10,13(18)-tetraene-17,19-dione |
|---|
| SMILES | COC1=CC2=C(C3=C1C1=C(C(=O)OCC1)C(=O)O3)C1(O)CCOC1O2 |
|---|
| InChI Identifier | InChI=1S/C17H14O8/c1-21-8-6-9-12(17(20)3-5-23-16(17)24-9)13-10(8)7-2-4-22-14(18)11(7)15(19)25-13/h6,16,20H,2-5H2,1H3 |
|---|
| InChI Key | OZSVZTQMDLNGFU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as difurocoumarolactones. These are polycyclic aromatic compounds containing a delta-valerolactone ring fused to the coumarin moiety of the difurocoumarin skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | Difurocoumarolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Difurocoumarolactone
- Difurocoumarin
- Benzopyran
- 1-benzopyran
- Coumaran
- Anisole
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Pyran
- Heteroaromatic compound
- Tetrahydrofuran
- Tertiary alcohol
- Carboxylic acid ester
- Lactone
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Ether
- Monocarboxylic acid or derivatives
- Alcohol
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gbi-0049000000-723b1668ff0de95b7f87 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dr-2009000000-abce4446bd82d1d673bd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-7b1eccce8a5207bc5745 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0029000000-2b1a36ae0e77602c8fd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000t-1090000000-5fb9897d469a847b8b3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-1bd081568743b34aa787 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0029000000-064b0c1fc8e9d3eeb13b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00p3-1190000000-de4758ebdfc7a9cb503e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-10a0e1c5c35fd491778a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0029000000-c147be58de6d73d691e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00or-0139000000-e4b061e48a566e56ea88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-24b80c82c41a998b9ea5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0009000000-d677bb8ccf0e8f8d23b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0059000000-7417f6434212aaeb1328 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030478 |
|---|
| FooDB ID | FDB002347 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013207 |
|---|
| ChEBI ID | 175363 |
|---|
| PubChem Compound ID | 131751028 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|