| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:43:32 UTC |
|---|
| Update Date | 2016-11-09 01:17:54 UTC |
|---|
| Accession Number | CHEM024476 |
|---|
| Identification |
|---|
| Common Name | Goldinodox |
|---|
| Class | Small Molecule |
|---|
| Description | Poultry growth promote |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
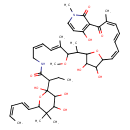
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methylmocimycin, 9ci | HMDB | | Antibiotic X 5108 | HMDB | | ANTIBIOTIC X-5108 | HMDB | | Aurodox | HMDB | | Goldinomycin | HMDB | | MAU | HMDB | | N-Methyl kirromycin | HMDB | | X 5108 | HMDB | | N-[(2Z,4E)-7-{3,4-dihydroxy-5-[(1Z,3E,5Z)-7-(4-hydroxy-1-methyl-2-oxo-1,2-dihydropyridin-3-yl)-6-methyl-7-oxohepta-1,3,5-trien-1-yl]oxolan-2-yl}-6-methoxy-5-methylocta-2,4-dien-1-yl]-2-{2,3,4-trihydroxy-5,5-dimethyl-6-[(1E,3Z)-penta-1,3-dien-1-yl]oxan-2-yl}butanimidate | HMDB | | N Methylkirromycin | HMDB | | N-Methylkirromycin | HMDB |
|
|---|
| Chemical Formula | C44H62N2O12 |
|---|
| Average Molecular Mass | 810.969 g/mol |
|---|
| Monoisotopic Mass | 810.430 g/mol |
|---|
| CAS Registry Number | 12704-90-4 |
|---|
| IUPAC Name | N-[(2Z,4E)-7-{3,4-dihydroxy-5-[(1Z,3E,5Z)-7-(4-hydroxy-1-methyl-2-oxo-1,2-dihydropyridin-3-yl)-6-methyl-7-oxohepta-1,3,5-trien-1-yl]oxolan-2-yl}-6-methoxy-5-methylocta-2,4-dien-1-yl]-2-{2,3,4-trihydroxy-5,5-dimethyl-6-[(1E,3Z)-penta-1,3-dien-1-yl]oxan-2-yl}butanamide |
|---|
| Traditional Name | N-[(2Z,4E)-7-{3,4-dihydroxy-5-[(1Z,3E,5Z)-7-(4-hydroxy-1-methyl-2-oxopyridin-3-yl)-6-methyl-7-oxohepta-1,3,5-trien-1-yl]oxolan-2-yl}-6-methoxy-5-methylocta-2,4-dien-1-yl]-2-{2,3,4-trihydroxy-5,5-dimethyl-6-[(1E,3Z)-penta-1,3-dien-1-yl]oxan-2-yl}butanamide |
|---|
| SMILES | CCC(C(=O)NC\C=C/C=C(\C)C(OC)C(C)C1OC(\C=C/C=C/C=C(/C)C(=O)C2=C(O)C=CN(C)C2=O)C(O)C1O)C1(O)OC(\C=C\C=C/C)C(C)(C)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C44H62N2O12/c1-10-12-14-22-32-43(6,7)39(51)40(52)44(55,58-32)29(11-2)41(53)45-24-18-17-20-27(4)37(56-9)28(5)38-36(50)35(49)31(57-38)21-16-13-15-19-26(3)34(48)33-30(47)23-25-46(8)42(33)54/h10,12-23,25,28-29,31-32,35-40,47,49-52,55H,11,24H2,1-9H3,(H,45,53)/b12-10-,15-13+,18-17-,21-16-,22-14+,26-19-,27-20+ |
|---|
| InChI Key | NTAHMPNXQOYXSX-NJWHXGCDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | C-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-glycosyl compound
- Aryl ketone
- Pyridinone
- Hydroxypyridine
- Dihydropyridine
- Monosaccharide
- N-acyl-amine
- Oxane
- Hydropyridine
- Pyridine
- Alpha-branched alpha,beta-unsaturated-ketone
- Fatty acyl
- Fatty amide
- Alpha,beta-unsaturated ketone
- Tetrahydrofuran
- Vinylogous acid
- Vinylogous amide
- Acryloyl-group
- Enone
- Heteroaromatic compound
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Ketone
- Hemiacetal
- Lactam
- Carboxylic acid derivative
- Dialkyl ether
- Oxacycle
- Ether
- Azacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Carbonyl group
- Organic oxide
- Organic nitrogen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-6900000410-d950513c6d5b68f8db6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fbc-9712413400-34372573d7adefd4ea84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fb9-9200100000-45f46dc5ee522a88f022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00e9-0900000000-bc4b116be6d161c6ae9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1900010100-5a17009a605cedd82091 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-4900000000-663d4416b250081e692c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01pn-0343900110-84be14ca1af91702bacc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f83-2480823910-cb44dfd315d5162a901c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-2940200000-a6cb37e4161a99a8be6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0059020130-9d118a546ac5b39be4f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-0192100100-27910dfa77b80e9620e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0frf-5393171010-9b6bfe11d536f1605956 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030466 |
|---|
| FooDB ID | FDB002335 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00018857 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013205 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 137185903 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|