| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:41:17 UTC |
|---|
| Update Date | 2016-11-09 01:17:53 UTC |
|---|
| Accession Number | CHEM024419 |
|---|
| Identification |
|---|
| Common Name | Simmondsin |
|---|
| Class | Small Molecule |
|---|
| Description | Simmondsin is found in coffee and coffee products. Simmondsin is a constituent of Simmondsia chinensis (jojoba) Simmondsin is an extract of jojoba seeds (pronounced "ho-HO-bah") (Simmondsia chinensis), it was traditionally thought to be a toxic substance due to jojoba seed meal causing weight loss in animals, but recently it has been researched as a potential treatment for reducing appetite of obese individuals by helping to reduce craving for food. Several mechanisms of action are thought to be involved in the appetite suppressant effect |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
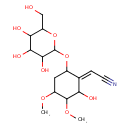
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Cyanomethylene-1-glucosyloxy-3-hydroxy-4,5-dimethoxycyclohexane | HMDB | | [6-(b-D-Glucopyranosyloxy)-2-hydroxy-3,4-dimethoxycyclohexylidene]acetonitrile, 9ci | HMDB | | 2-(Cyanomethylene)-3-hydroxy-4,5-dimethoxycyclohexyl-beta-glucoside | HMDB | | Simmondsin | MeSH |
|
|---|
| Chemical Formula | C16H25NO9 |
|---|
| Average Molecular Mass | 375.371 g/mol |
|---|
| Monoisotopic Mass | 375.153 g/mol |
|---|
| CAS Registry Number | 51771-52-9 |
|---|
| IUPAC Name | 2-[(1E)-2-hydroxy-3,4-dimethoxy-6-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}cyclohexylidene]acetonitrile |
|---|
| Traditional Name | 2-[(1E)-2-hydroxy-3,4-dimethoxy-6-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}cyclohexylidene]acetonitrile |
|---|
| SMILES | COC1CC(OC2OC(CO)C(O)C(O)C2O)\C(=C\C#N)C(O)C1OC |
|---|
| InChI Identifier | InChI=1S/C16H25NO9/c1-23-9-5-8(7(3-4-17)11(19)15(9)24-2)25-16-14(22)13(21)12(20)10(6-18)26-16/h3,8-16,18-22H,5-6H2,1-2H3/b7-3- |
|---|
| InChI Key | KURSRHBVYUACKS-CLTKARDFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- O-glycosyl compound
- Cyclitol or derivatives
- Monosaccharide
- Oxane
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Acetal
- Organoheterocyclic compound
- Nitrile
- Carbonitrile
- Oxacycle
- Ether
- Dialkyl ether
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Alcohol
- Primary alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pbc-6709000000-6998950778cd28d8115f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0002-5700029000-7d5f45c590134067edbc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08i1-0859000000-75c9aa237be28bb87161 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0931000000-334b1b9ad66bce406d80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-9810000000-7e46d7dfb818bceb9ef2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0229-1549000000-f17a64c9d81a11e6b273 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-2942000000-754398f69da1c15e0816 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0295-5930000000-dc664cfc0e9ff444576b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0119000000-d8c528e451fd3e295a78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-0933000000-3b292e8d59637d682a1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fr-7690000000-c0bd49d4c6ff3a7fbb04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0019000000-a564b8e7ffa695a32dd3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0c00-3879000000-f826e8fc4fa488e85bb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-8941000000-de6b200cc3b38374a3e6 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030394 |
|---|
| FooDB ID | FDB002248 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054414 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Simmondsin |
|---|
| Chemspider ID | 4733931 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5884740 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|