| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:40:39 UTC |
|---|
| Update Date | 2016-11-09 01:17:53 UTC |
|---|
| Accession Number | CHEM024402 |
|---|
| Identification |
|---|
| Common Name | (E)-4-Methoxy-3,3',5,5'-tetrahydroxystilbene |
|---|
| Class | Small Molecule |
|---|
| Description | (Z)-4-Methoxy-3,3',5,5'-tetrahydroxystilbene is found in fruits. (Z)-4-Methoxy-3,3',5,5'-tetrahydroxystilbene is isolated from Phoenix dactylifera (date). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
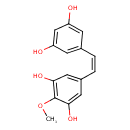
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,3',5,5'-Tetrahydroxy-4-methoxystilbene | HMDB | | 4-Methoxy-3,3',5,5'-tetrahydroxystilbene | HMDB | | 5-[2-(3,5-Dihydroxyphenyl)ethenyl]-2-methoxy-1,3-benzenediol | HMDB |
|
|---|
| Chemical Formula | C15H14O5 |
|---|
| Average Molecular Mass | 274.269 g/mol |
|---|
| Monoisotopic Mass | 274.084 g/mol |
|---|
| CAS Registry Number | 89946-11-2 |
|---|
| IUPAC Name | 5-[(Z)-2-(3,5-dihydroxyphenyl)ethenyl]-2-methoxybenzene-1,3-diol |
|---|
| Traditional Name | 5-[(Z)-2-(3,5-dihydroxyphenyl)ethenyl]-2-methoxybenzene-1,3-diol |
|---|
| SMILES | COC1=C(O)C=C(\C=C/C2=CC(O)=CC(O)=C2)C=C1O |
|---|
| InChI Identifier | InChI=1S/C15H14O5/c1-20-15-13(18)6-10(7-14(15)19)3-2-9-4-11(16)8-12(17)5-9/h2-8,16-19H,1H3/b3-2- |
|---|
| InChI Key | BKJYMZRGLINXRP-IHWYPQMZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Resorcinol
- Styrene
- Methoxybenzene
- 1-hydroxy-4-unsubstituted benzenoid
- Phenol
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Ether
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-0390000000-2336ae0c8f211504b1fc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0002-1000590000-79925140283b97cdbc38 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-7d50c958a4010ff8f64f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0490000000-5e2583126d8263efe84d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05n0-1930000000-de4fed1070fe3855b73a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-a49c13c4f76119f9629f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-a71a0292b851bd5b533e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1590000000-523a51b41c3bafa8c3f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-e2a03e643571c4d09086 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0490000000-6833d18cd27dbb6a04f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-1930000000-fcc0a31a35338b899b3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-7dbb1e549f6404042b70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0290000000-6b0f9ab02553a0d0e716 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1930000000-6bc6102a12bd9c4de781 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030377 |
|---|
| FooDB ID | FDB012020 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00015561 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10298689 |
|---|
| ChEBI ID | 174600 |
|---|
| PubChem Compound ID | 13499471 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|