| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:40:00 UTC |
|---|
| Update Date | 2016-11-09 01:17:53 UTC |
|---|
| Accession Number | CHEM024385 |
|---|
| Identification |
|---|
| Common Name | (-)-Aspidospermine |
|---|
| Class | Small Molecule |
|---|
| Description | An indole alkaloid having the structure of aspirospermidine methoxylated at C-17 and acetylated at N-1. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
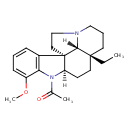
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Acetyl-17-methoxyaspidospermidine | ChEBI | | 1-(17-Methoxyaspidospermidin-1-yl)-ethanone | HMDB | | 1-Acetyl-17-methoxyaspidospermidine, 9ci | HMDB | | (-)-Aspidospermine | ChEBI |
|
|---|
| Chemical Formula | C22H30N2O2 |
|---|
| Average Molecular Mass | 354.486 g/mol |
|---|
| Monoisotopic Mass | 354.231 g/mol |
|---|
| CAS Registry Number | 466-49-9 |
|---|
| IUPAC Name | 1-[(1R,9R,12R,19R)-12-ethyl-6-methoxy-8,16-diazapentacyclo[10.6.1.0¹,⁹.0²,⁷.0¹⁶,¹⁹]nonadeca-2(7),3,5-trien-8-yl]ethan-1-one |
|---|
| Traditional Name | aspidospermine |
|---|
| SMILES | [H][C@@]12CC[C@@]3(CC)CCCN4CC[C@]1(C1=C(N2C(C)=O)C(OC)=CC=C1)[C@@]34[H] |
|---|
| InChI Identifier | InChI=1S/C22H30N2O2/c1-4-21-10-6-13-23-14-12-22(20(21)23)16-7-5-8-17(26-3)19(16)24(15(2)25)18(22)9-11-21/h5,7-8,18,20H,4,6,9-14H2,1-3H3/t18-,20-,21-,22-/m1/s1 |
|---|
| InChI Key | ARQOGCYMPUOVHK-ZHHKINOHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aspidospermatan-type alkaloids. These are tryptophan-derived alkaloids that are derived from the fusion of tryptamine and a terpene unit (generally either 9 or 10 carbons). Aspidospermine and aspidospermidine (along with tabersonine) are the archetypical members of the Aspidosperma alkaloids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aspidospermatan-type alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aspidospermatan-type alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspidosperma alkaloid
- Plumeran-type alkaloid
- Carbazole
- Quinolidine
- Indole or derivatives
- Indolizidine
- Anisole
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Benzenoid
- N-alkylpyrrolidine
- Acetamide
- Tertiary carboxylic acid amide
- Pyrrolidine
- Amino acid or derivatives
- Carboxamide group
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Ether
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Amine
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01tl-1029000000-96af6a463ae68aa05f3f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-c0f2e042065aee132604 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-0029000000-4dde02d5047aec262fe9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053e-1092000000-a85ba17b2078c2965803 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-65e65f5bd7e74a4d526e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w29-1029000000-89bdefe0c1c9697de5fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-2098000000-645f445fb2c7b32cccdc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-240bf3ec656a869d732e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w29-0009000000-1b64cc789cf3df742940 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udj-0059000000-827fd6ef228d38dfaf6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-f7af0f531d28f8508f59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0009000000-f7af0f531d28f8508f59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-0229000000-306014f44651374af804 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030361 |
|---|
| FooDB ID | FDB002207 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024465 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 198027 |
|---|
| ChEBI ID | 28463 |
|---|
| PubChem Compound ID | 227613 |
|---|
| Kegg Compound ID | C09042 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|