| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:39:58 UTC |
|---|
| Update Date | 2016-11-09 01:17:53 UTC |
|---|
| Accession Number | CHEM024384 |
|---|
| Identification |
|---|
| Common Name | (+)-Aspidospermidine |
|---|
| Class | Small Molecule |
|---|
| Description | (+)-Aspidospermidine is an alkaloid from Aspidosperma quebracho-blanco (quebracho |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
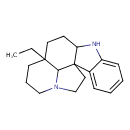
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dehydrodivanillyl alcohol | HMDB | | Aspidospermidine | HMDB |
|
|---|
| Chemical Formula | C19H26N2 |
|---|
| Average Molecular Mass | 282.423 g/mol |
|---|
| Monoisotopic Mass | 282.210 g/mol |
|---|
| CAS Registry Number | 2912-09-6 |
|---|
| IUPAC Name | 12-ethyl-8,16-diazapentacyclo[10.6.1.0¹,⁹.0²,⁷.0¹⁶,¹⁹]nonadeca-2,4,6-triene |
|---|
| Traditional Name | 12-ethyl-8,16-diazapentacyclo[10.6.1.0¹,⁹.0²,⁷.0¹⁶,¹⁹]nonadeca-2,4,6-triene |
|---|
| SMILES | CCC12CCCN3CCC4(C(CC1)NC1=CC=CC=C41)C23 |
|---|
| InChI Identifier | InChI=1S/C19H26N2/c1-2-18-9-5-12-21-13-11-19(17(18)21)14-6-3-4-7-15(14)20-16(19)8-10-18/h3-4,6-7,16-17,20H,2,5,8-13H2,1H3 |
|---|
| InChI Key | YAAIPCQYJYPITK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aspidospermatan-type alkaloids. These are tryptophan-derived alkaloids that are derived from the fusion of tryptamine and a terpene unit (generally either 9 or 10 carbons). Aspidospermine and aspidospermidine (along with tabersonine) are the archetypical members of the Aspidosperma alkaloids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aspidospermatan-type alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aspidospermatan-type alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspidosperma alkaloid
- Plumeran-type alkaloid
- Carbazole
- Quinolidine
- Indole or derivatives
- Dihydroindole
- Indolizidine
- Aralkylamine
- Secondary aliphatic/aromatic amine
- Benzenoid
- N-alkylpyrrolidine
- Piperidine
- Pyrrolidine
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organonitrogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-1090000000-c7bb8d6d49a21a981379 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-753254926ca73b72c086 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-2d7e9eab8bc37eaeec6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-0690000000-716fdd0d3058d52a0b96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-d5dae2723d86ad3781cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-6510ef744feb6b1c41a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gbi-1090000000-a528bcd16e1da0dca7d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-4af417b6c8cdf87c31f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-4af417b6c8cdf87c31f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0090000000-ee911222c1051ced6521 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-48c8d6d60cf70e82e98f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-8a1139910294cabb38b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-2590000000-0841c2cbd400320fd4b4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030360 |
|---|
| FooDB ID | FDB002206 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024464 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 504390 |
|---|
| ChEBI ID | 174012 |
|---|
| PubChem Compound ID | 580281 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Stevens MM, Ali A, Helliwell S, Schiller LJ, Hansen S: Comparison of two bioassay techniques for assessing the acute toxicity of pesticides to chironomid larvae (Diptera: Chironomidae). J Am Mosq Control Assoc. 2002 Jun;18(2):119-25. | | 2. Wang L, Xu K, Bei F, Gao FS: [Effects of bagging on the microenvironment, yield and quality of overwintering tomato]. Ying Yong Sheng Tai Xue Bao. 2007 Apr;18(4):837-42. | | 3. Laskowski DA: Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol. 2002;174:49-170. | | 4. Rodriguez MM, Bisset JA, Fernandez D: Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 2007 Dec;23(4):420-9. | | 5. Ahmad M, Arif MI, Denholm I: High resistance of field populations of the cotton aphid Aphis gossypii Glover (Homoptera: Aphididae) to pyrethroid insecticides in Pakistan. J Econ Entomol. 2003 Jun;96(3):875-8. | | 6. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|