| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:39:52 UTC |
|---|
| Update Date | 2016-11-09 01:17:53 UTC |
|---|
| Accession Number | CHEM024380 |
|---|
| Identification |
|---|
| Common Name | 14,19-Didehydrocondyfolan |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from Aspidosperma quebracho-blanco (quebracho). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
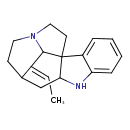
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 14,19-Didehydro-(14E)-condyfolan | HMDB | | 14,19-Didehydrocondyfolan, 9ci | HMDB |
|
|---|
| Chemical Formula | C18H22N2 |
|---|
| Average Molecular Mass | 266.381 g/mol |
|---|
| Monoisotopic Mass | 266.178 g/mol |
|---|
| CAS Registry Number | 3890-05-9 |
|---|
| IUPAC Name | (18E)-18-ethylidene-8,14-diazapentacyclo[9.5.2.0¹,⁹.0²,⁷.0¹⁴,¹⁷]octadeca-2,4,6-triene |
|---|
| Traditional Name | (18E)-18-ethylidene-8,14-diazapentacyclo[9.5.2.0¹,⁹.0²,⁷.0¹⁴,¹⁷]octadeca-2,4,6-triene |
|---|
| SMILES | C\C=C1\C2N3CCC22C(CC1CC3)NC1=CC=CC=C21 |
|---|
| InChI Identifier | InChI=1S/C18H22N2/c1-2-13-12-7-9-20-10-8-18(17(13)20)14-5-3-4-6-15(14)19-16(18)11-12/h2-6,12,16-17,19H,7-11H2,1H3/b13-2+ |
|---|
| InChI Key | DSEPQHRHLTVWHD-XNJYKOPJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as strychnos alkaloids. These are alkaloids having a core structure based on the strychnan, stemmadenine (seco-curan), or the akuammicine (curan) skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Strychnos alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Strychnos alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspidosperma alkaloid
- Stemmadenine-skeleton
- Carbazole
- Indole or derivatives
- Dihydroindole
- Indolizidine
- Aralkylamine
- Secondary aliphatic/aromatic amine
- Piperidine
- Benzenoid
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary amine
- Tertiary aliphatic amine
- Secondary amine
- Organoheterocyclic compound
- Azacycle
- Amine
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-1090000000-912fbaf5f371d5834ba1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-3edbbad8cd8b5ed48fa6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-2ebaa5db81dfb1b7a6e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0g4r-0960000000-59c99163ffc3b5c4643f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-352ed3b33d6d2c8f71a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-37bf8378786f7ebad244 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kk-0090000000-2c5c4c2a34df3889485c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-3eabb2a4cedb6ab1b611 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-3eabb2a4cedb6ab1b611 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0190000000-35ae22d7364df7536d6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-eaa4946cc9c73719d0b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-eaa4946cc9c73719d0b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0090000000-a748636073991b87e926 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030356 |
|---|
| FooDB ID | FDB002202 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751004 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|