| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:39:37 UTC |
|---|
| Update Date | 2016-11-09 01:17:53 UTC |
|---|
| Accession Number | CHEM024372 |
|---|
| Identification |
|---|
| Common Name | Anonaine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from Annona muricata (soursop) and Nelumbo nucifera (East India lotus). Anonaine is found in many foods, some of which are sugar apple, sacred lotus, fruits, and custard apple. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
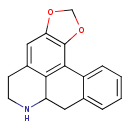
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Annonaine | HMDB | | 1,2-Methylenedioxynoraporphine | HMDB | | Anonain | HMDB |
|
|---|
| Chemical Formula | C17H15NO2 |
|---|
| Average Molecular Mass | 265.307 g/mol |
|---|
| Monoisotopic Mass | 265.110 g/mol |
|---|
| CAS Registry Number | 1862-41-5 |
|---|
| IUPAC Name | 3,5-dioxa-11-azapentacyclo[10.7.1.0²,⁶.0⁸,²⁰.0¹⁴,¹⁹]icosa-1(20),2(6),7,14,16,18-hexaene |
|---|
| Traditional Name | 3,5-dioxa-11-azapentacyclo[10.7.1.0²,⁶.0⁸,²⁰.0¹⁴,¹⁹]icosa-1(20),2(6),7,14,16,18-hexaene |
|---|
| SMILES | C1OC2=C(O1)C1=C3C(CC4=CC=CC=C14)NCCC3=C2 |
|---|
| InChI Identifier | InChI=1S/C17H15NO2/c1-2-4-12-10(3-1)7-13-15-11(5-6-18-13)8-14-17(16(12)15)20-9-19-14/h1-4,8,13,18H,5-7,9H2 |
|---|
| InChI Key | VZTUKBKUWSHDFM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aporphines. These are quinoline alkaloids containing the dibenzo[de,g]quinoline ring system or a dehydrogenated derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aporphines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aporphines |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kr-0290000000-eef0189b19c023e62512 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-e9a2afab256159ae2699 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-f7ca3522e83754962ac6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00pl-1980000000-a0014a6236a9bf51d76e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-74185903329bd522c04a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-7de3784592381bb7a862 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014m-1490000000-2f75c30aa943164353af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-4ceb63c2dd5efc77f44c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-4ceb63c2dd5efc77f44c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ds-0190000000-9a10adc3866934e256bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-f67efedff389fe87473b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-e7468aac66a5ed55954f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-0090000000-d457525b01f969160b5d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030348 |
|---|
| FooDB ID | FDB002193 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027281 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Anonaine |
|---|
| Chemspider ID | 2704037 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3462225 |
|---|
| Kegg Compound ID | C09339 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|