| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:37:21 UTC |
|---|
| Update Date | 2016-11-09 01:17:52 UTC |
|---|
| Accession Number | CHEM024305 |
|---|
| Identification |
|---|
| Common Name | (-)-Chimonanthine |
|---|
| Class | Small Molecule |
|---|
| Description | A ring assembly that is 2,2',3,3',8,8',8a,8a'-octahydro-1H,1'H-3a,3a'-bipyrrolo[2,3-b]indole substituted by methyl groups at positions 1 and 1'. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
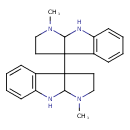
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Demethyl-calycanthidine | HMDB | | 1-Demethylcalycanthidine | HMDB | | Chimonanthin | HMDB | | Chimonanthine | HMDB | | L-Chimonanthine | HMDB |
|
|---|
| Chemical Formula | C22H26N4 |
|---|
| Average Molecular Mass | 346.469 g/mol |
|---|
| Monoisotopic Mass | 346.216 g/mol |
|---|
| CAS Registry Number | 5545-89-1 |
|---|
| IUPAC Name | 1-methyl-3a-{1-methyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-3a-yl}-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indole |
|---|
| Traditional Name | chimonanthine |
|---|
| SMILES | CN1CCC2(C1NC1=C2C=CC=C1)C12CCN(C)C1NC1=C2C=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C22H26N4/c1-25-13-11-21(15-7-3-5-9-17(15)23-19(21)25)22-12-14-26(2)20(22)24-18-10-6-4-8-16(18)22/h3-10,19-20,23-24H,11-14H2,1-2H3 |
|---|
| InChI Key | HOYXPMHLHJOGHD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrroloindoles. Pyrroloindoles are compounds containing a pyrroloindole moiety, which is a tricyclic heterocycle which consists of a pyrrole ring fused to an indole. Pyrrole is 5-membered ring consisting of four carbon atoms and one nitrogen atom. Indole is a bicyclic compound consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Pyrroloindoles |
|---|
| Direct Parent | Pyrroloindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrroloindole
- Indole
- Dihydroindole
- Secondary aliphatic/aromatic amine
- Benzenoid
- N-alkylpyrrolidine
- Pyrrole
- Pyrrolidine
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fz9-0839000000-e6dc51fe7686225cb76e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-ede6dda140966de044ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1009000000-5fc4e48dea2309927723 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001r-6094000000-72a46e04248bd284193c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0209000000-f04b1a47b3ee880c297d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1109000000-036750f7127d1ff0f027 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0avi-2934000000-45e537f8de9ae517804d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-37c95b847a9e45a22589 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0019000000-5f6508ee1c8e805d7483 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdx-2695000000-2bc392ec5723c1849154 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-a5ed12fd7e24b3ac19f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-a5ed12fd7e24b3ac19f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0902000000-eaebb7fc5a7a7f5c48bf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030280 |
|---|
| FooDB ID | FDB002115 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00026840 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 520787 |
|---|
| ChEBI ID | 38955 |
|---|
| PubChem Compound ID | 599086 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|