| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:36:52 UTC |
|---|
| Update Date | 2016-11-09 01:17:52 UTC |
|---|
| Accession Number | CHEM024294 |
|---|
| Identification |
|---|
| Common Name | Anomurine |
|---|
| Class | Small Molecule |
|---|
| Description | Anomurine is found in fruits. Minor alkaloid from the root and stem bark of Annona muricata (soursop |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
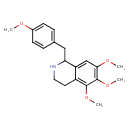
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3,4-Tetrahydro-5,6,7-trimethoxy-1-[(4-methoxyphenyl)methyl]isoquinoline, 9ci | HMDB |
|
|---|
| Chemical Formula | C20H25NO4 |
|---|
| Average Molecular Mass | 343.417 g/mol |
|---|
| Monoisotopic Mass | 343.178 g/mol |
|---|
| CAS Registry Number | 78478-27-0 |
|---|
| IUPAC Name | 5,6,7-trimethoxy-1-[(4-methoxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline |
|---|
| Traditional Name | 5,6,7-trimethoxy-1-[(4-methoxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline |
|---|
| SMILES | COC1=CC=C(CC2NCCC3=C(OC)C(OC)=C(OC)C=C23)C=C1 |
|---|
| InChI Identifier | InChI=1S/C20H25NO4/c1-22-14-7-5-13(6-8-14)11-17-16-12-18(23-2)20(25-4)19(24-3)15(16)9-10-21-17/h5-8,12,17,21H,9-11H2,1-4H3 |
|---|
| InChI Key | CMQSBRRTRZPLHE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzylisoquinolines. These are organic compounds containing an isoquinoline to which a benzyl group is attached. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoquinolines and derivatives |
|---|
| Sub Class | Benzylisoquinolines |
|---|
| Direct Parent | Benzylisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzylisoquinoline
- Tetrahydroisoquinoline
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Secondary aliphatic amine
- Ether
- Azacycle
- Secondary amine
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0895000000-136e2644ca82a9894238 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-9bae7ea3e4375132926e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vo-0669000000-6db2ed0b82fd74e85eb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5l-0950000000-feca7fe187cabe356124 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-15d00f4264564f5bf359 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-0019000000-d8ddef0135e27882c033 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uld-1190000000-2a0bef587c83f83ff7aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-5a516184c230fa2e7a58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0049000000-6c1dec2f50f28c760550 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0196000000-d43ad39675720536faa5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-d805c0618b333d81d510 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0019000000-4498cc26f5293b4aa535 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01vk-0391000000-14e4c8e126e2284ee591 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030270 |
|---|
| FooDB ID | FDB002101 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00052470 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 138367 |
|---|
| ChEBI ID | 219219 |
|---|
| PubChem Compound ID | 157218 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|