| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:36:50 UTC |
|---|
| Update Date | 2016-11-09 01:17:52 UTC |
|---|
| Accession Number | CHEM024293 |
|---|
| Identification |
|---|
| Common Name | Nigellicine |
|---|
| Class | Small Molecule |
|---|
| Description | Nigellicine is found in herbs and spices. Nigellicine is an alkaloid from the seeds of Nigella sativa (black cumin |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
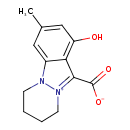
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Methyl-1-oxo-1H,6H,7H,8H,9H-pyridazino[1,2-a]indazole-11-carboxylate | HMDB | | Nigellicine | MeSH |
|
|---|
| Chemical Formula | C13H14N2O3 |
|---|
| Average Molecular Mass | 246.262 g/mol |
|---|
| Monoisotopic Mass | 246.100 g/mol |
|---|
| CAS Registry Number | 98063-20-8 |
|---|
| IUPAC Name | 1-hydroxy-3-methyl-6H,7H,8H,9H-10λ⁵-pyridazino[1,2-a]indazol-10-ylium-11-carboxylate |
|---|
| Traditional Name | 1-hydroxy-3-methyl-6H,7H,8H,9H-10λ⁵-pyridazino[1,2-a]indazol-10-ylium-11-carboxylate |
|---|
| SMILES | CC1=CC(O)=C2C(=C1)N1CCCC[N+]1=C2C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C13H14N2O3/c1-8-6-9-11(10(16)7-8)12(13(17)18)15-5-3-2-4-14(9)15/h6-7H,2-5H2,1H3,(H,17,18) |
|---|
| InChI Key | FEJTUHSIRAJLOJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridazinoindazoles. Pyridazinoindazoles are compounds containing a pyridazinoindazole moiety, which consists of a pyridazine ring fused to an indazole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrazoles |
|---|
| Sub Class | Indazoles |
|---|
| Direct Parent | Pyridazinoindazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridazinoindazole
- Pyrazole-5-carboxylic acid or derivatives
- Pyrazole-3-carboxylic acid or derivatives
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Pyridazine
- Azole
- Heteroaromatic compound
- Pyrazole
- Carboxylic acid salt
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organic nitrogen compound
- Organic zwitterion
- Organic salt
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0il0-0590000000-6921f5972e7773a50da9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fk9-3394000000-9e8975148d1e663333ce | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0090000000-978fa98900985f1e5ce2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fba-0090000000-e0709a3844b163789814 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f9f-4980000000-4f325fe25660252482d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0090000000-74ba8bd2b056292c5754 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0290000000-c48d0a6b94b8e48afdfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1940000000-63a5e0edd4fbb43be935 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udj-0090000000-ba92633025182ce5ec98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0290000000-10f4eee6f4dc25824cab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-015a-0910000000-c38f54b8d3a193a04433 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-4b96f2fb9c6435e34917 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-360167aec658ca76c222 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cki-0910000000-e9c09a512e2e3d46c03e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030269 |
|---|
| FooDB ID | FDB002099 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00028686 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9577233 |
|---|
| ChEBI ID | 168947 |
|---|
| PubChem Compound ID | 11402337 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|