| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:36:49 UTC |
|---|
| Update Date | 2016-11-09 01:17:52 UTC |
|---|
| Accession Number | CHEM024292 |
|---|
| Identification |
|---|
| Common Name | Dumetorine |
|---|
| Class | Small Molecule |
|---|
| Description | Dumetorine is found in root vegetables. Dumetorine is an alkaloid from the tubers of the famine food Dioscorea dumetoru |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
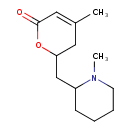
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5,6-Dihydro-4-methyl-6-[(1-methyl-2-piperidinyl)methyl]-2H-pyran-2-one, 9ci | HMDB | | Dumetorine | MeSH |
|
|---|
| Chemical Formula | C13H21NO2 |
|---|
| Average Molecular Mass | 223.311 g/mol |
|---|
| Monoisotopic Mass | 223.157 g/mol |
|---|
| CAS Registry Number | 96552-67-9 |
|---|
| IUPAC Name | 4-methyl-6-[(1-methylpiperidin-2-yl)methyl]-5,6-dihydro-2H-pyran-2-one |
|---|
| Traditional Name | 4-methyl-6-[(1-methylpiperidin-2-yl)methyl]-5,6-dihydropyran-2-one |
|---|
| SMILES | CN1CCCCC1CC1CC(C)=CC(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C13H21NO2/c1-10-7-12(16-13(15)8-10)9-11-5-3-4-6-14(11)2/h8,11-12H,3-7,9H2,1-2H3 |
|---|
| InChI Key | JFFVCKNYHMIHTF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydropyranones. Dihydropyranones are compounds containing a hydrogenated pyran ring which bears a ketone, and contains one double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrans |
|---|
| Sub Class | Pyranones and derivatives |
|---|
| Direct Parent | Dihydropyranones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydropyranone
- Piperidine
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Amino acid or derivatives
- Carboxylic acid ester
- Lactone
- Tertiary amine
- Tertiary aliphatic amine
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Azacycle
- Oxacycle
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Amine
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9100000000-e43ef4c02e08c358df24 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0229-0890000000-f350310de411976db586 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vt-8910000000-e59d0efbcc00cd255ed7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xu-9300000000-3f36caa18face9122fdc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-0690000000-74521ef8480f97c39eb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fr-3980000000-7f28694054bfa5b83e59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5m-9500000000-93f51be67e09b293290f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0390000000-6178797bcea259ebefd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00b9-3930000000-5a16f878967a563e2917 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aos-9400000000-bfa90d85b9f8293935b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dj-4290000000-3e0de19f1ac4c4350b40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-074j-5970000000-c7c148273111c10f7467 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05u2-9100000000-79d6e86e296e5c25b668 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030268 |
|---|
| FooDB ID | FDB002098 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054333 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013169 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13858447 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|