| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:36:06 UTC |
|---|
| Update Date | 2016-11-09 01:17:51 UTC |
|---|
| Accession Number | CHEM024272 |
|---|
| Identification |
|---|
| Common Name | (-)-Norushinsunine |
|---|
| Class | Small Molecule |
|---|
| Description | (-)-norushinsunine is a member of the class of compounds known as hydroxy-7-aporphines. Hydroxy-7-aporphines are aporphines containing a hydroxyl group at the 7-position (-)-norushinsunine is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). (-)-norushinsunine can be found in cherimoya, custard apple, and sugar apple, which makes (-)-norushinsunine a potential biomarker for the consumption of these food products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
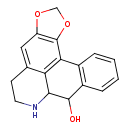
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C17H15NO3 |
|---|
| Average Molecular Mass | 281.306 g/mol |
|---|
| Monoisotopic Mass | 281.105 g/mol |
|---|
| CAS Registry Number | 3175-84-6 |
|---|
| IUPAC Name | 3,5-dioxa-11-azapentacyclo[10.7.1.0²,⁶.0⁸,²⁰.0¹⁴,¹⁹]icosa-1(20),2(6),7,14(19),15,17-hexaen-13-ol |
|---|
| Traditional Name | 3,5-dioxa-11-azapentacyclo[10.7.1.0²,⁶.0⁸,²⁰.0¹⁴,¹⁹]icosa-1(20),2(6),7,14(19),15,17-hexaen-13-ol |
|---|
| SMILES | OC1C2NCCC3=CC4=C(OCO4)C(C4=C1C=CC=C4)=C23 |
|---|
| InChI Identifier | InChI=1S/C17H15NO3/c19-16-11-4-2-1-3-10(11)14-13-9(5-6-18-15(13)16)7-12-17(14)21-8-20-12/h1-4,7,15-16,18-19H,5-6,8H2 |
|---|
| InChI Key | CKIYSMRPIBQTHQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxy-7-aporphines. These are aporphines containing a hydroxyl group at the 7-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aporphines |
|---|
| Sub Class | Hydroxy-7-aporphines |
|---|
| Direct Parent | Hydroxy-7-aporphines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxy-7-aporphine
- Benzoquinoline
- Phenanthrene
- Naphthalene
- Quinoline
- Tetrahydroisoquinoline
- Benzodioxole
- Aralkylamine
- Benzenoid
- 1,2-aminoalcohol
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Secondary aliphatic amine
- Acetal
- Secondary amine
- Organic oxygen compound
- Amine
- Organic nitrogen compound
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0090000000-37e6b842589bcb0a9ad5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0090000000-eff1c3da0c904505fe2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2790000000-db33095b516fa8835e84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-2d8bb661bca93a062ccd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-0090000000-819801ca69d129d77e23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06sl-2190000000-6a7fe41f9adfca0f8b93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-cd1939612c9ef5597ee0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-712beeee8df97ec94593 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ta-0090000000-ead0133d2345b2d48a49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-3b4cac44e39678d48174 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-cbdce8f67132e70cc8b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0090000000-a46810c466cd3b43caa1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302139 |
|---|
| FooDB ID | FDB002073 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4478038 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5319820 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|