| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:35:19 UTC |
|---|
| Update Date | 2016-11-09 01:17:51 UTC |
|---|
| Accession Number | CHEM024252 |
|---|
| Identification |
|---|
| Common Name | Lagerstroemine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from Plantago psyllium seeds (African plantain). Lagerstroemine is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
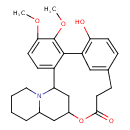
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Indicamine? | HMDB | | Lagerstremine | HMDB |
|
|---|
| Chemical Formula | C26H31NO5 |
|---|
| Average Molecular Mass | 437.528 g/mol |
|---|
| Monoisotopic Mass | 437.220 g/mol |
|---|
| CAS Registry Number | 10247-53-7 |
|---|
| IUPAC Name | 9-hydroxy-5,6-dimethoxy-16-oxa-24-azapentacyclo[15.7.1.1⁸,¹².0²,⁷.0¹⁹,²⁴]hexacosa-2,4,6,8,10,12(26)-hexaen-15-one |
|---|
| Traditional Name | 9-hydroxy-5,6-dimethoxy-16-oxa-24-azapentacyclo[15.7.1.1⁸,¹².0²,⁷.0¹⁹,²⁴]hexacosa-2,4,6,8,10,12(26)-hexaen-15-one |
|---|
| SMILES | COC1=CC=C2C3CC(CC4CCCCN34)OC(=O)CCC3=CC(=C(O)C=C3)C2=C1OC |
|---|
| InChI Identifier | InChI=1S/C26H31NO5/c1-30-23-10-8-19-21-15-18(14-17-5-3-4-12-27(17)21)32-24(29)11-7-16-6-9-22(28)20(13-16)25(19)26(23)31-2/h6,8-10,13,17-18,21,28H,3-5,7,11-12,14-15H2,1-2H3 |
|---|
| InChI Key | RYMSMTOEKVDTDB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as macrolides and analogues. These are organic compounds containing a lactone ring of at least twelve members. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolides and analogues |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Macrolides and analogues |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4j-5005900000-66e98964fe933f291b3a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-000w-5000900000-f2237fa7f9fee3e39c39 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0002900000-7ec1698e708f832b8b48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0018900000-67013c2236879047cf01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0041-6942000000-3b34ceddafb9b3b1b9cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0001900000-69e31ce0ec11d2e45e30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-0113900000-72b8a3d535c57a0f2752 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9004300000-8680e1e18192dd4f9499 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-f26cc601530dc8904f7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000900000-c61347fab788da054a1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fl9-0009600000-d731d709afc5e0bdef48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-41e0b1ec119bcc0d930d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0000900000-ed93d928ff0275f04a85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9005200000-297a965b7bd51b2e0d62 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030229 |
|---|
| FooDB ID | FDB002048 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 78385190 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|