| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:35:16 UTC |
|---|
| Update Date | 2016-11-09 01:17:51 UTC |
|---|
| Accession Number | CHEM024251 |
|---|
| Identification |
|---|
| Common Name | Nigakinone |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from the wood of Picrasma excelsa (Jamaican quassiawood). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
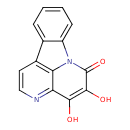
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,5-Dimethoxy-6H-indolo(3,2,1-de)(1,5)naphthyridin-6-one | HMDB | | 4,5-Dimethoxy-6H-indolo[3,2,1-de]-1,5-naphthyridin-6-one | HMDB | | 4,5-Dimethoxy-6H-indolo[3,2,1-de][1,5]naphthyridin-6-one | HMDB | | 5-Hydroxy-4-methoxy-6H-indolo[3,2,1-de][1,5]naphthyridin-6-one, 9ci | HMDB | | 5-Hydroxy-4-methoxycanthin-6-one | HMDB | | Methyl nigakinone | HMDB |
|
|---|
| Chemical Formula | C14H8N2O3 |
|---|
| Average Molecular Mass | 252.225 g/mol |
|---|
| Monoisotopic Mass | 252.053 g/mol |
|---|
| CAS Registry Number | 18110-86-6 |
|---|
| IUPAC Name | 3,4-dihydroxy-1,6-diazatetracyclo[7.6.1.0⁵,¹⁶.0¹⁰,¹⁵]hexadeca-3,5,7,9(16),10,12,14-heptaen-2-one |
|---|
| Traditional Name | 3,4-dihydroxy-1,6-diazatetracyclo[7.6.1.0⁵,¹⁶.0¹⁰,¹⁵]hexadeca-3,5,7,9(16),10,12,14-heptaen-2-one |
|---|
| SMILES | OC1=C(O)C2=NC=CC3=C2N(C2=CC=CC=C32)C1=O |
|---|
| InChI Identifier | InChI=1S/C14H8N2O3/c17-12-10-11-8(5-6-15-10)7-3-1-2-4-9(7)16(11)14(19)13(12)18/h1-6,17-18H |
|---|
| InChI Key | XPMYKCPEDQIDCM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolonaphthyridine alkaloids. These are a numerous and relatively straightforward subgroup of the b-carbolines, e.g. Canthin-6-one, in which an additional C3 unit is attached between C-1 and the indole nitrogen to form an additional ring. The group includes a few dimeric examples, such as Haplophytine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Indolonaphthyridine alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Indolonaphthyridine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolo[3,2-1de][1,5]naphthyridine
- Beta-carboline
- Pyridoindole
- Diazanaphthalene
- Naphthyridine
- Indole
- Indole or derivatives
- Indolizine
- Pyrrolopyridine
- Hydroxypyridine
- Pyridinone
- Benzenoid
- Pyridine
- Vinylogous acid
- Heteroaromatic compound
- Pyrrole
- Lactam
- Organoheterocyclic compound
- Azacycle
- Organic oxide
- Organonitrogen compound
- Organic oxygen compound
- Organooxygen compound
- Organopnictogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0390000000-310c3ad20c019421e703 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00e9-3119000000-2f52b75ab4ef56a40c11 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-ffd4ff1b19deb16890a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0090000000-c87f98c9d0149a1aa4de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0900000000-d5b59f6be190aca23a65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0290000000-3a9284f6f8845f17905e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0590000000-8650ba377405918ee49f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-46d0ce11b863d9811348 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-eb7c2ff7d3762b310f84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-c3a3700c91cde77e4c6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-0900000000-fc190407fe92bb1193ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-bff44a744dc6d7c2d865 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0090000000-bff44a744dc6d7c2d865 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-0930000000-6b3731402c27a24cf814 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030228 |
|---|
| FooDB ID | FDB002047 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776821 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 137185901 |
|---|
| Kegg Compound ID | C16996 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|