| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:34:29 UTC |
|---|
| Update Date | 2016-11-09 01:17:51 UTC |
|---|
| Accession Number | CHEM024227 |
|---|
| Identification |
|---|
| Common Name | (-)-Fumigaclavine B |
|---|
| Class | Small Molecule |
|---|
| Description | (-)-Fumigaclavine B is a mycotoxin from Aspergillus fumigatus and Rhizopus arrhizu |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
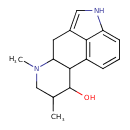
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Demethylfrangulanine | HMDB | | Fumigaclavine b | HMDB | | 6,8-Dimethylergolin-9-ol | HMDB | | Isofumigaclavine b | HMDB |
|
|---|
| Chemical Formula | C16H20N2O |
|---|
| Average Molecular Mass | 256.343 g/mol |
|---|
| Monoisotopic Mass | 256.158 g/mol |
|---|
| CAS Registry Number | 6879-93-2 |
|---|
| IUPAC Name | 4,6-dimethyl-6,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(16),9,12,14-tetraen-3-ol |
|---|
| Traditional Name | 4,6-dimethyl-6,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(16),9,12,14-tetraen-3-ol |
|---|
| SMILES | CC1CN(C)C2CC3=CNC4=CC=CC(C2C1O)=C34 |
|---|
| InChI Identifier | InChI=1S/C16H20N2O/c1-9-8-18(2)13-6-10-7-17-12-5-3-4-11(14(10)12)15(13)16(9)19/h3-5,7,9,13,15-17,19H,6,8H2,1-2H3 |
|---|
| InChI Key | JUXRVSRUBIFVKE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as clavines and derivatives. These are hydroxy and dehydro derivatives of 6,8-dimethylergolenes and the corresponding ergolines. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Ergoline and derivatives |
|---|
| Sub Class | Clavines and derivatives |
|---|
| Direct Parent | Clavines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Clavine skeleton
- Indoloquinoline
- Benzoquinoline
- Pyrroloquinoline
- Quinoline
- 3-alkylindole
- Indole
- Indole or derivatives
- Isoindole or derivatives
- Aralkylamine
- Benzenoid
- Piperidine
- Pyrrole
- Heteroaromatic compound
- Secondary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-1920000000-81fed3643e2843d2b1f1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006t-7963000000-2c120c45120ede6301af | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0090000000-d859a3cf6e1c9345396e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0390000000-13481dda933b6e9ac3d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-0900000000-b35006c40391c11a8811 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-1b660ecea89bd9325ef5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1290000000-d07330c7897fca721935 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-3900000000-1a8188063faf0b18fcb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0090000000-97d8d65a48f025f64a37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0090000000-d7017d8926ef3a2cac18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03ni-0940000000-00b87fbb8c4f9f1015a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-ca82db7f0f7f6291e478 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-ef72f227b8673d1b10e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004r-0890000000-c960f8bbaaa20800cc46 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030201 |
|---|
| FooDB ID | FDB002019 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00011248 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013159 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12309937 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|