| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:34:19 UTC |
|---|
| Update Date | 2016-11-09 01:17:51 UTC |
|---|
| Accession Number | CHEM024225 |
|---|
| Identification |
|---|
| Common Name | Frangulanine |
|---|
| Class | Small Molecule |
|---|
| Description | Frangulanine is found in fruits. Frangulanine is an alkaloid from the root bark of Ceanothus americanus (New Jersey tea), Hovenia dulcis (raisin tree) and Zizyphus jujuba var. inermi |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
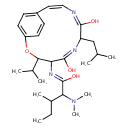
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(Dimethylamino)-3-methyl-N-[3-(1-methylethyl)-7-(2-methylpropyl)-5,8-dioxo-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-10,12,14,15-tetraen-4-yl]pentanamide, 9ci | HMDB | | Ceanothamine a | HMDB | | Daechuine S2 | HMDB | | N-[(10Z)-5,8-Dihydroxy-7-(2-methylpropyl)-3-(propan-2-yl)-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-2-(dimethylamino)-3-methylpentanimidate | HMDB | | Frangulanine | MeSH |
|
|---|
| Chemical Formula | C28H44N4O4 |
|---|
| Average Molecular Mass | 500.673 g/mol |
|---|
| Monoisotopic Mass | 500.336 g/mol |
|---|
| CAS Registry Number | 25350-22-5 |
|---|
| IUPAC Name | (Z)-N-[(5E,8E,10Z)-5,8-dihydroxy-7-(2-methylpropyl)-3-(propan-2-yl)-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-2-(dimethylamino)-3-methylpentimidic acid |
|---|
| Traditional Name | (Z)-N-[(5E,8E,10Z)-5,8-dihydroxy-3-isopropyl-7-(2-methylpropyl)-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-2-(dimethylamino)-3-methylpentimidic acid |
|---|
| SMILES | CCC(C)C(N(C)C)C(\O)=N\C1C(OC2=CC=C(C=C2)\C=C/N=C(O)\C(CC(C)C)\N=C1\O)C(C)C |
|---|
| InChI Identifier | InChI=1S/C28H44N4O4/c1-9-19(6)24(32(7)8)28(35)31-23-25(18(4)5)36-21-12-10-20(11-13-21)14-15-29-26(33)22(16-17(2)3)30-27(23)34/h10-15,17-19,22-25H,9,16H2,1-8H3,(H,29,33)(H,30,34)(H,31,35)/b15-14- |
|---|
| InChI Key | ULQXKOIGVXLOOC-PFONDFGASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Isoleucine or derivatives
- N-acyl-alpha amino acid or derivatives
- Macrolactam
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Alkyl aryl ether
- N-acyl-amine
- Fatty amide
- Fatty acyl
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Amino acid or derivatives
- Oxacycle
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0btc-9300200000-e79d2fd28e9e6648165b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0bt9-9300002000-28c04d045f8b060ee374 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-114i-0509370000-2a4a9b8fe261b80a9618 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08fr-4809000000-306ac7b273887873a3b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cka-9102000000-e2377b3e1a050d5e6494 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0101900000-d6357db6a82d431d3158 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-1305900000-ce612174b3f0176d0008 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0btc-8409000000-425602382ba3185399e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-0009700000-d64303c8bc9b85499e05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-0009200000-9213bf40c5dc768a4b60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2019000000-fc0ec1045717ff9c42b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000090000-714375b6bd04706159a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-2303290000-96fac2404794e400d58e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-114j-5209000000-f64f73da7e736567f0be | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030199 |
|---|
| FooDB ID | FDB002017 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001998 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4476260 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5317388 |
|---|
| Kegg Compound ID | C10003 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|