| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:33:43 UTC |
|---|
| Update Date | 2016-11-09 01:17:51 UTC |
|---|
| Accession Number | CHEM024207 |
|---|
| Identification |
|---|
| Common Name | Erythratine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from the seeds of Erythrina glauca (gallito). Erythratine is found in green vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
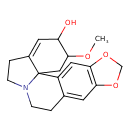
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,6-Didehydro-3-methoxy-15,16-[methylenebis(oxy)]erythrinan-2-ol, 9ci | HMDB |
|
|---|
| Chemical Formula | C18H21NO4 |
|---|
| Average Molecular Mass | 315.364 g/mol |
|---|
| Monoisotopic Mass | 315.147 g/mol |
|---|
| CAS Registry Number | 5550-20-9 |
|---|
| IUPAC Name | 19-methoxy-5,7-dioxa-13-azapentacyclo[11.7.0.0¹,¹⁶.0²,¹⁰.0⁴,⁸]icosa-2,4(8),9,16-tetraen-18-ol |
|---|
| Traditional Name | 19-methoxy-5,7-dioxa-13-azapentacyclo[11.7.0.0¹,¹⁶.0²,¹⁰.0⁴,⁸]icosa-2,4(8),9,16-tetraen-18-ol |
|---|
| SMILES | COC1CC23N(CCC2=CC1O)CCC1=CC2=C(OCO2)C=C31 |
|---|
| InChI Identifier | InChI=1S/C18H21NO4/c1-21-17-9-18-12(7-14(17)20)3-5-19(18)4-2-11-6-15-16(8-13(11)18)23-10-22-15/h6-8,14,17,20H,2-5,9-10H2,1H3 |
|---|
| InChI Key | ZIAIDGYDHGYWBH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as erythrinanes. These are erythrina alkaloids possessing either a 6-5-6-6-membered indoloisoquinoline core or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Erythrina alkaloids |
|---|
| Sub Class | Erythrinanes |
|---|
| Direct Parent | Erythrinanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Erythrinane skeleton
- Tetrahydroisoquinoline
- Benzodioxole
- Indole or derivatives
- Aralkylamine
- Benzenoid
- N-alkylpyrrolidine
- Pyrrolidine
- Secondary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Oxacycle
- Dialkyl ether
- Ether
- Azacycle
- Acetal
- Organoheterocyclic compound
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01t9-1090000000-9cd4cf79ce23bfbadc35 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-024i-8197000000-e19e4017d53cd32d4040 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0039000000-9205b955c498a6f200a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0095000000-733520a7d0c129d6f784 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fbc-0290000000-0e2a6170b2dbcb93ed8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0029000000-10c7bdc4d4ddffd29689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1097000000-11c6f1aedae1f3bba625 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mk-3090000000-01ad606cea224e556995 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-a9f6ec6e6fe2a282293c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0059000000-84c3094f8869db38bf17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ta-0091000000-47209987c6645dcbe456 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-135924c69299cfc5fa80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0059000000-dce2672b4f4a5e37d393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0191000000-0adce574bfeb3992f6e5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030181 |
|---|
| FooDB ID | FDB001997 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00030212 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 536982 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 617879 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|