| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:33:26 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024198 |
|---|
| Identification |
|---|
| Common Name | Oxyacanthine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from Berberis vulgaris (barberry). Oxyacanthine is found in tea and fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
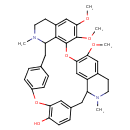
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6,6',7-Trimethoxy-2,2'-dimethyloxyacanthan-12'-ol | HMDB | | 6,6'7'-Trimethoxy-2,2'-dimethyl-oxyacanthan-12'-ol | HMDB | | 6,6'7-Trimethoxy-2,2'-dimethyl-oxyacanthan-12'-ol | HMDB | | N-Methylocoteamine | HMDB | | N-Methylsepeerine | HMDB | | Oxycanthine | HMDB |
|
|---|
| Chemical Formula | C37H40N2O6 |
|---|
| Average Molecular Mass | 608.723 g/mol |
|---|
| Monoisotopic Mass | 608.289 g/mol |
|---|
| CAS Registry Number | 548-40-3 |
|---|
| IUPAC Name | 20,21,25-trimethoxy-15,30-dimethyl-8,23-dioxa-15,30-diazaheptacyclo[22.6.2.2⁹,¹².1³,⁷.1¹⁴,¹⁸.0²⁷,³¹.0²²,³³]hexatriaconta-3(36),4,6,9,11,18,20,22(33),24,26,31,34-dodecaen-6-ol |
|---|
| Traditional Name | 20,21,25-trimethoxy-15,30-dimethyl-8,23-dioxa-15,30-diazaheptacyclo[22.6.2.2⁹,¹².1³,⁷.1¹⁴,¹⁸.0²⁷,³¹.0²²,³³]hexatriaconta-3(36),4,6,9,11,18,20,22(33),24,26,31,34-dodecaen-6-ol |
|---|
| SMILES | COC1=C2OC3=C4C(CC5=CC=C(OC6=C(O)C=CC(CC7N(C)CCC(=C1)C7=C2)=C6)C=C5)N(C)CCC4=CC(OC)=C3OC |
|---|
| InChI Identifier | InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(41-3)33-21-27(24)28(38)17-23-8-11-30(40)31(18-23)44-26-9-6-22(7-10-26)16-29-35-25(13-15-39(29)2)20-34(42-4)36(43-5)37(35)45-33/h6-11,18-21,28-29,40H,12-17H2,1-5H3 |
|---|
| InChI Key | HGNHIFJNOKGSKI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lignans, neolignans and related compounds. These are plant products of low molecular weight formed primarily from oxidative coupling of two p-propylphenol moieties. They can also be described as micromolecules with two phenylpropanoid units coupled together. They can be attached in various manners, like C5-C5', C8-C8'. Most known natural lignans are oxidized at C9 and C9´ and, based upon the way in which oxygen is incorporated into the skeleton and on the cyclization patterns, a wide range of lignans of very different structural types can be formed. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lignans, neolignans and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxyneolignan skeleton
- Diaryl ether
- Tetrahydroisoquinoline
- Anisole
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Amine
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-0000090000-c412b15bd9537b35a376 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01b9-1000009000-1223820c9148dc5e06c5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Oxyacanthine,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-0a4i-0000009000-28831833ea904387d520 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-0a4i-0000009000-38aaab7723d1f732dda6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000039000-2bad9850c5dc0f081dd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a7i-0000093000-2790909e7f0f30709acf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0j4j-0000090000-25372cf1899667ad464e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000009000-62bbbb52d6800c881d5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-0000097000-49657eb97feeda86a735 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-0000190000-9c83502b2c4a0e62bf78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000009000-243a991931ec2986982a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0000094000-c16ffa8f9e1a6a7a1b98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0000090000-baf9852cd591be14f779 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000009000-5debb5ccf794890680ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000059000-9d376161adb6686be389 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pec-1000090000-60cd43712b70897111e5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030172 |
|---|
| FooDB ID | FDB001988 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001897 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 329538 |
|---|
| ChEBI ID | 7853 |
|---|
| PubChem Compound ID | 371257 |
|---|
| Kegg Compound ID | C09598 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|