| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:33:24 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024197 |
|---|
| Identification |
|---|
| Common Name | N-Methyl-14-O-demethylepiporphyroxine |
|---|
| Class | Small Molecule |
|---|
| Description | N-Methyl-14-O-demethylepiporphyroxine is an alkaloid from Papaver somniferum (opium poppy |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
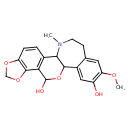
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Glycerol tri-a-eleostearate | HMDB | | Tri-a-eleostearoylglycerol | HMDB | | Alkaloid a4 | HMDB |
|
|---|
| Chemical Formula | C20H21NO6 |
|---|
| Average Molecular Mass | 371.384 g/mol |
|---|
| Monoisotopic Mass | 371.137 g/mol |
|---|
| CAS Registry Number | 18361-67-6 |
|---|
| IUPAC Name | 17-methoxy-22-methyl-6,8,12-trioxa-22-azapentacyclo[11.9.0.0²,¹⁰.0⁵,⁹.0¹⁴,¹⁹]docosa-2(10),3,5(9),14,16,18-hexaene-11,16-diol |
|---|
| Traditional Name | 17-methoxy-22-methyl-6,8,12-trioxa-22-azapentacyclo[11.9.0.0²,¹⁰.0⁵,⁹.0¹⁴,¹⁹]docosa-2(10),3,5(9),14,16,18-hexaene-11,16-diol |
|---|
| SMILES | COC1=C(O)C=C2C3OC(O)C4=C(C=CC5=C4OCO5)C3N(C)CCC2=C1 |
|---|
| InChI Identifier | InChI=1S/C20H21NO6/c1-21-6-5-10-7-15(24-2)13(22)8-12(10)18-17(21)11-3-4-14-19(26-9-25-14)16(11)20(23)27-18/h3-4,7-8,17-18,20,22-23H,5-6,9H2,1-2H3 |
|---|
| InChI Key | DPRSKEMBOBQDJV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as rhoeadine alkaloids. These are alkaloids with a structure based on rhoeadine. They usually contain a benzazepine system fused with a six-membered hemiacetal or acetal. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Rhoeadine alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Rhoeadine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Rhoeadine-skeleton
- Benzazepine
- Benzopyran
- Isochromane
- 2-benzopyran
- Benzodioxole
- Anisole
- Alkyl aryl ether
- Aralkylamine
- Azepine
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Tertiary aliphatic amine
- Hemiacetal
- Tertiary amine
- Azacycle
- Ether
- Oxacycle
- Acetal
- Organoheterocyclic compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organopnictogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-0948000000-00e8f85ff31ddc53d034 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0ufr-1090550000-f61af1feaa5b85f1f3d4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0019000000-93d4491b49d0c6ad72a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0019000000-131e9b19e2ae65f8aaef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01vp-6498000000-074f5481774e64813e0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-75f594a97642babc547b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0009000000-b3bdaf6bdbcdb22e04aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-0019000000-656ebc7d7ef963ce220c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-3c5e15c1d773a911db1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0009000000-890c6f3c12dce6a22d9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udj-4922000000-82d8e09eb5fd784db729 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-e8c3c11ee0a59cbca6a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0009000000-54572ca245f9fa254c42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-0289000000-8e653a96f98872ff7ccf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030171 |
|---|
| FooDB ID | FDB001987 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055112 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013155 |
|---|
| ChEBI ID | 175773 |
|---|
| PubChem Compound ID | 122376203 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|