| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:33:09 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024193 |
|---|
| Identification |
|---|
| Common Name | Euglobal III |
|---|
| Class | Small Molecule |
|---|
| Description | From blackcurrant (Ribes nigrum) (Grossulariaceae). Dehydroxymethylflazine is found in fruits and blackcurrant. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
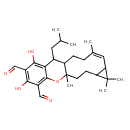
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(2-Furanyl)-9H-pyrido[3,4-b]indole-3-carboxylic acid | HMDB | | 1-(2-Furyl)-9H-pyrido(3,4-b)indole-3-carboxylic acid | HMDB | | 1-(2-Furyl)pyrido(3,4-b)indole-3-carboxylic acid | HMDB |
|
|---|
| Chemical Formula | C28H38O5 |
|---|
| Average Molecular Mass | 454.598 g/mol |
|---|
| Monoisotopic Mass | 454.272 g/mol |
|---|
| CAS Registry Number | 76449-26-8 |
|---|
| IUPAC Name | (7Z)-14,16-dihydroxy-1,5,5,8-tetramethyl-12-(2-methylpropyl)-19-oxatetracyclo[9.8.0.0⁴,⁶.0¹³,¹⁸]nonadeca-7,13,15,17-tetraene-15,17-dicarbaldehyde |
|---|
| Traditional Name | (7Z)-14,16-dihydroxy-1,5,5,8-tetramethyl-12-(2-methylpropyl)-19-oxatetracyclo[9.8.0.0⁴,⁶.0¹³,¹⁸]nonadeca-7,13,15,17-tetraene-15,17-dicarbaldehyde |
|---|
| SMILES | CC(C)CC1C2CC\C(C)=C/C3C(CCC2(C)OC2=C(C=O)C(O)=C(C=O)C(O)=C12)C3(C)C |
|---|
| InChI Identifier | InChI=1S/C28H38O5/c1-15(2)11-17-20-8-7-16(3)12-22-21(27(22,4)5)9-10-28(20,6)33-26-19(14-30)24(31)18(13-29)25(32)23(17)26/h12-15,17,20-22,31-32H,7-11H2,1-6H3/b16-12- |
|---|
| InChI Key | AGTXIGWLMDUUMQ-VBKFSLOCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as harmala alkaloids. Harmala alkaloids are compounds with a structure based on harmaline, harmine, harmalol, harman or a derivative of those parents. These parents are beta-carbolines, consisting of a pyrimidine fused to the pyrrole moiety of an indole to form a pyrido[3,4-b]indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Harmala alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Harmala alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Harman
- Beta-carboline
- Pyridoindole
- Indole
- Indole or derivatives
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Pyridine
- Benzenoid
- Furan
- Heteroaromatic compound
- Pyrrole
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Azacycle
- Carboxylic acid
- Oxacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p6-4004900000-710eac6bdb9ef75699f2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001l-2300190000-b3e51877c51315996421 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0020900000-740d4bc6eb77f98da978 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-3252900000-7a41d2a1ca4c9f82767a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktr-9652300000-e6f971189a1e34a67f37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-4c95937e69632ab37c54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000900000-c0bdb743985b48df99ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02du-3317900000-dcfd9c36f90788fd4762 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-0db53eae8610a0e47ec3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000900000-1f8e05fa0ece55daaca1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-114u-4098700000-3f052720719abf0aa170 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0001900000-ff91c1653b202db6aaba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0901-1026900000-0829213f2e9d84b63ce3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-9228600000-8c458a645354b7ff9a52 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041200 |
|---|
| FooDB ID | FDB021099 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4589673 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5488120 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|