| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:32:48 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024182 |
|---|
| Identification |
|---|
| Common Name | Austalide I |
|---|
| Class | Small Molecule |
|---|
| Description | Mycotoxin from the food storage mould (Aspergillus ustus). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
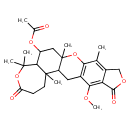
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| GLY-leu-tyr | HMDB | | GLYCYL-L-leucyl-L-tyrosine | HMDB | | GLYCYL-leucyl-tyrosine | HMDB | | N-(N-GLYCYL-L-leucyl)-L-tyrosine | HMDB | | 10-Methoxy-1,4,14,19,19-pentamethyl-8,17-dioxo-2,7,18-trioxapentacyclo[11.9.0.0³,¹¹.0⁵,⁹.0¹⁴,²⁰]docosa-3(11),4,9-trien-21-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C27H34O8 |
|---|
| Average Molecular Mass | 486.554 g/mol |
|---|
| Monoisotopic Mass | 486.225 g/mol |
|---|
| CAS Registry Number | 96817-08-2 |
|---|
| IUPAC Name | 10-methoxy-1,4,14,19,19-pentamethyl-8,17-dioxo-2,7,18-trioxapentacyclo[11.9.0.0³,¹¹.0⁵,⁹.0¹⁴,²⁰]docosa-3(11),4,9-trien-21-yl acetate |
|---|
| Traditional Name | 10-methoxy-1,4,14,19,19-pentamethyl-8,17-dioxo-2,7,18-trioxapentacyclo[11.9.0.0³,¹¹.0⁵,⁹.0¹⁴,²⁰]docosa-3(11),4,9-trien-21-yl acetate |
|---|
| SMILES | COC1=C2C(=O)OCC2=C(C)C2=C1CC1C(C)(CC(OC(C)=O)C3C1(C)CCC(=O)OC3(C)C)O2 |
|---|
| InChI Identifier | InChI=1S/C27H34O8/c1-13-16-12-32-24(30)20(16)22(31-7)15-10-18-26(5)9-8-19(29)34-25(3,4)23(26)17(33-14(2)28)11-27(18,6)35-21(13)15/h17-18,23H,8-12H2,1-7H3 |
|---|
| InChI Key | XBPVWACQUMEORV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthenes. These are polycyclic aromatic compounds containing a xanthene moiety, which consists of two benzene rings joined to each other by a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Xanthenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthene
- Isobenzofuranone
- Phthalide
- Isocoumaran
- Tricarboxylic acid or derivatives
- Anisole
- Caprolactone
- Alkyl aryl ether
- Oxepane
- Benzenoid
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Oxacycle
- Ether
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01xx-2011900000-7e76da0b18af8cd98fb4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0041900000-d6b78baa4d9004b047a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0072900000-3cc83f2495196cc43cb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r03-4690300000-049cdc8a7cdb2a6cd526 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-0001900000-482b7799789f257fd76e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-054o-2011900000-c750d73268dbba64ef0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9212300000-be5f888b49dab1a39ed8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1000900000-ead074036aacbb22b92d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-fbcedc0a649031858791 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9001200000-5521b3f16bbcc416e186 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000900000-fc3c7a316a18bd8593a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00p0-0010900000-038abfac2e30d91ba725 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05p6-4022900000-4a80e8b118523fed65a4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030155 |
|---|
| FooDB ID | FDB001964 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131750972 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|