| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:32:37 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024176 |
|---|
| Identification |
|---|
| Common Name | Jasmine ketolactone |
|---|
| Class | Small Molecule |
|---|
| Description | Jasmine ketolactone is found in herbs and spices. Jasmine ketolactone is a constituent of Italian jasmine oil (Jasminum grandiflorum) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
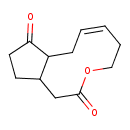
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1R,7Z,10S)-4-Oxabicyclo[8.3.0]tridec-7-ene-3,11-dione | HMDB | | 1,4,5,8,8a,10,11,11a-Octahydrocyclopent[D]oxecin-2,9-dione, 9ci | HMDB | | 5'-Hydroxyjasmonic acid lactone | HMDB | | Jasmine oil ketolactone | HMDB |
|
|---|
| Chemical Formula | C12H16O3 |
|---|
| Average Molecular Mass | 208.254 g/mol |
|---|
| Monoisotopic Mass | 208.110 g/mol |
|---|
| CAS Registry Number | 70981-24-7 |
|---|
| IUPAC Name | 1H,2H,4H,5H,8H,8aH,9H,10H,11H,11aH-cyclopenta[d]oxecine-2,9-dione |
|---|
| Traditional Name | 1H,4H,5H,8H,8aH,10H,11H,11aH-cyclopenta[d]oxecine-2,9-dione |
|---|
| SMILES | O=C1CCC2CC(=O)OCC\C=C/CC12 |
|---|
| InChI Identifier | InChI=1S/C12H16O3/c13-11-6-5-9-8-12(14)15-7-3-1-2-4-10(9)11/h1-2,9-10H,3-8H2/b2-1- |
|---|
| InChI Key | DINQMNROFIPFOH-UPHRSURJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxocins. Oxocins are compounds containing an oxocin ring, which is a eight-member unsaturated aromatic ring containing one oxygen atom and seven carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxocins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Oxocins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxocin
- Lactone
- Ketone
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uxr-2900000000-c3e21ea3434d2f1fc7ab | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0790000000-768dc90cc91a7cb42f67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0693-4920000000-ee74478677c909bafa3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9100000000-33dc22ebb942fce2a30c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0290000000-704901fd52c1b8d36a69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4950000000-e59091e25c06faad9d45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0btd-9600000000-c378ed1f699070ac3795 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-65fdf834be31e8e78310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0290000000-2bf335c68736debf0c7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0920000000-3082ce6f00ee0500c8aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0970000000-7dc3c2c4cc4d74d6e54e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-0930000000-3229b9e0fa69249c6d46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03xs-0900000000-e43c592846e8022e56ea | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030150 |
|---|
| FooDB ID | FDB001958 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057513 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 19240450 |
|---|
| ChEBI ID | 173593 |
|---|
| PubChem Compound ID | 21134279 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|