| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:31:59 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024156 |
|---|
| Identification |
|---|
| Common Name | gamma-Pyrufuran |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Pyrus communis (pear) infected with Chondrostereum purpureum. gamma-Pyrufuran is found in pomes. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
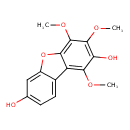
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| g-Pyrufuran | Generator | | Γ-pyrufuran | Generator | | 1,3,4-Trimethoxydibenzofuran-2,7-diol | HMDB | | 2,7-Dihydroxy-1,3,4-trimethoxydibenzofuran | HMDB |
|
|---|
| Chemical Formula | C15H14O6 |
|---|
| Average Molecular Mass | 290.268 g/mol |
|---|
| Monoisotopic Mass | 290.079 g/mol |
|---|
| CAS Registry Number | 93973-18-3 |
|---|
| IUPAC Name | 3,5,6-trimethoxy-8-oxatricyclo[7.4.0.0²,⁷]trideca-1(13),2,4,6,9,11-hexaene-4,11-diol |
|---|
| Traditional Name | 3,5,6-trimethoxy-8-oxatricyclo[7.4.0.0²,⁷]trideca-1(13),2,4,6,9,11-hexaene-4,11-diol |
|---|
| SMILES | COC1=C2OC3=CC(O)=CC=C3C2=C(OC)C(O)=C1OC |
|---|
| InChI Identifier | InChI=1S/C15H14O6/c1-18-12-10-8-5-4-7(16)6-9(8)21-13(10)15(20-3)14(19-2)11(12)17/h4-6,16-17H,1-3H3 |
|---|
| InChI Key | KHGAPBYQBHBCBX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzofurans. Dibenzofurans are compounds containing a dibenzofuran moiety, which consists of two benzene rings fused to a central furan ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzofurans |
|---|
| Sub Class | Dibenzofurans |
|---|
| Direct Parent | Dibenzofurans |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03fr-0090000000-e5c0e520a09b26c730a6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-3027900000-cdfda035a728a04720c8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-46fedc22d8d4a7060b31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-49d458a252ef8e15a41b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002b-1090000000-399c5db40319dd121862 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-634ace74f3bbbba42f23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-f652d001c7700a6f0bb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-4490000000-8c3842dfbd00ed5ea62a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-afe43bdc82c44950ba4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-afe43bdc82c44950ba4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004r-1950000000-f7979623c85da0e99d1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-2d980c515d025d58a6fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-ddc3323ca742fb591752 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-2490000000-42aeaa1ba87bfdb1b4d5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030131 |
|---|
| FooDB ID | FDB001935 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 161099 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 185310 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|