| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:30:54 UTC |
|---|
| Update Date | 2016-11-09 01:17:50 UTC |
|---|
| Accession Number | CHEM024126 |
|---|
| Identification |
|---|
| Common Name | Methyl [8]-Shogaol |
|---|
| Class | Small Molecule |
|---|
| Description | Methyl [8]-Shogaol is found in ginger. Methyl [8]-Shogaol is isolated from ginger (Zingiber officinale) [DFC] (Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
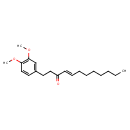
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(3,4-Dimethoxyphenyl)-4-dodecen-3-one | HMDB |
|
|---|
| Chemical Formula | C20H30O3 |
|---|
| Average Molecular Mass | 318.450 g/mol |
|---|
| Monoisotopic Mass | 318.219 g/mol |
|---|
| CAS Registry Number | 863780-79-4 |
|---|
| IUPAC Name | (4E)-1-(3,4-dimethoxyphenyl)dodec-4-en-3-one |
|---|
| Traditional Name | (4E)-1-(3,4-dimethoxyphenyl)dodec-4-en-3-one |
|---|
| SMILES | CCCCCCC\C=C\C(=O)CCC1=CC(OC)=C(OC)C=C1 |
|---|
| InChI Identifier | InChI=1S/C20H30O3/c1-4-5-6-7-8-9-10-11-18(21)14-12-17-13-15-19(22-2)20(16-17)23-3/h10-11,13,15-16H,4-9,12,14H2,1-3H3/b11-10+ |
|---|
| InChI Key | ZNOLGYFCFIVHQI-ZHACJKMWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dimethoxybenzenes. These are organic aromatic compounds containing a monocyclic benzene moiety carrying exactly two methoxy groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Methoxybenzenes |
|---|
| Direct Parent | Dimethoxybenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-dimethoxybenzene
- Dimethoxybenzene
- Phenoxy compound
- Anisole
- Phenol ether
- Alkyl aryl ether
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Enone
- Ketone
- Ether
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-9530000000-ff7106e3214b3c36c5d9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0109000000-5d2f7a5d2d2b92523c91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0839000000-15ff598fbd0016f6fbd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07xr-0950000000-e080d2624ab9cc468ac1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-062ad2a35e11d303cf23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0739000000-f9367f29d39d819b5c06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ap1-4930000000-be548142feac860506c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0219000000-fea1b4d6682637438943 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-4912000000-a5236ad5c2521b86c3a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9510000000-65bd76dec197fc086a60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0309000000-1f98098939b52b3681c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gb9-5935000000-556cad5f5e5dc442747b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uy0-2900000000-da47fe1a8501edb01090 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030101 |
|---|
| FooDB ID | FDB001899 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00035689 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776809 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 91721121 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Afendi FM, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, Ikeda S, Takahashi H, Altaf-Ul-Amin M, Darusman LK, Saito K, Kanaya S. (2012) KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2012 Feb;53(2):e1. |
|
|---|