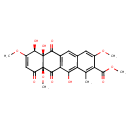| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:29:53 UTC |
|---|
| Update Date | 2016-11-09 01:17:49 UTC |
|---|
| Accession Number | CHEM024095 |
|---|
| Identification |
|---|
| Common Name | Foeniculoside X |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C24H22O11 |
|---|
| Average Molecular Mass | 486.425 g/mol |
|---|
| Monoisotopic Mass | 486.116 g/mol |
|---|
| CAS Registry Number | 946617-64-7 |
|---|
| IUPAC Name | methyl (6aR,7S,10aR)-6a,7,12-trihydroxy-3,8,10a-trimethoxy-1-methyl-6,10,11-trioxo-6,6a,7,10,10a,11-hexahydrotetracene-2-carboxylate |
|---|
| Traditional Name | methyl (6aR,7S,10aR)-6a,7,12-trihydroxy-3,8,10a-trimethoxy-1-methyl-6,10,11-trioxo-7H-tetracene-2-carboxylate |
|---|
| SMILES | COC(=O)C1=C(C)C2=C(O)C3=C(C=C2C=C1OC)C(=O)[C@@]1(O)[C@H](O)C(OC)=CC(=O)[C@@]1(OC)C3=O |
|---|
| InChI Identifier | InChI=1S/C24H22O11/c1-9-15-10(7-12(32-2)16(9)22(30)34-4)6-11-17(18(15)26)21(29)24(35-5)14(25)8-13(33-3)20(28)23(24,31)19(11)27/h6-8,20,26,28,31H,1-5H3/t20-,23-,24-/m1/s1 |
|---|
| InChI Key | QSPIPUXWSNFXCK-AGILITTLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetracenequinones. These are polyaromatic hydrocarbon derivatives containing a tetracyclic cycle made up of four linearly fused benzene rings, one of which bears two ketone groups at position 1 and 4. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthacenes |
|---|
| Sub Class | Tetracenequinones |
|---|
| Direct Parent | Tetracenequinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetracenequinone
- Anthracene carboxylic acid or derivatives
- Anthracene carboxylic acid
- 1,4-anthraquinone
- 9,10-anthraquinone
- 2-naphthalenecarboxylic acid or derivatives
- 2-naphthalenecarboxylic acid
- 1-naphthol
- O-methoxybenzoic acid or derivatives
- Tetralin
- Naphthalene
- Aryl alkyl ketone
- Aryl ketone
- Quinone
- Anisole
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Cyclohexenone
- Vinylogous ester
- Vinylogous acid
- Methyl ester
- Tertiary alcohol
- Ketone
- Carboxylic acid ester
- 1,2-diol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Polyol
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-8214fecb9b8d358d8f4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0010900000-f0748ca032e2c1f4c909 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000m-2025900000-af8947ec3f12eb0b4de9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-972914cf472b839f2e20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000900000-ea5af6df1ff6bdf11b75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3023900000-0a8dd6456d03ce480478 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0000900000-b6c40843d2b92c70bbfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05di-0002900000-6a82a2e42521ebb6e650 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00g0-4359800000-204e04a6c45049791613 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-6a828bd457b661bfbaf1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0016900000-0018d49fddf65eee68c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053l-6006900000-a091b1d5921542f469b7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302109 |
|---|
| FooDB ID | FDB001867 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | OCW |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 114600 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 129395 |
|---|
| Kegg Compound ID | C12380 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|