| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:23:29 UTC |
|---|
| Update Date | 2016-11-09 01:17:48 UTC |
|---|
| Accession Number | CHEM023952 |
|---|
| Identification |
|---|
| Common Name | Tinctormine |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
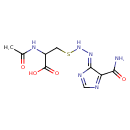
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-({2-[(4Z)-5-carbamoyl-4H-imidazol-4-ylidene]hydrazin-1-yl}sulfanyl)-2-[(1-hydroxyethylidene)amino]propanoate | Generator | | 3-({2-[(4Z)-5-carbamoyl-4H-imidazol-4-ylidene]hydrazin-1-yl}sulphanyl)-2-[(1-hydroxyethylidene)amino]propanoate | Generator | | 3-({2-[(4Z)-5-carbamoyl-4H-imidazol-4-ylidene]hydrazin-1-yl}sulphanyl)-2-[(1-hydroxyethylidene)amino]propanoic acid | Generator |
|
|---|
| Chemical Formula | C9H12N6O4S |
|---|
| Average Molecular Mass | 300.294 g/mol |
|---|
| Monoisotopic Mass | 300.064 g/mol |
|---|
| CAS Registry Number | 149475-43-4 |
|---|
| IUPAC Name | 3-({2-[(4Z)-5-carbamoyl-4H-imidazol-4-ylidene]hydrazin-1-yl}sulfanyl)-2-acetamidopropanoic acid |
|---|
| Traditional Name | 3-({2-[(4Z)-5-carbamoylimidazol-4-ylidene]hydrazin-1-yl}sulfanyl)-2-acetamidopropanoic acid |
|---|
| SMILES | CC(=O)NC(CSN\N=C1/N=CN=C1C(N)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H12N6O4S/c1-4(16)13-5(9(18)19)2-20-15-14-8-6(7(10)17)11-3-12-8/h3,5,15H,2H2,1H3,(H2,10,17)(H,13,16)(H,18,19)/b14-8- |
|---|
| InChI Key | PRJLAFYIJBKNFG-ZSOIEALJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-amino acid
- Cysteine or derivatives
- Imidazolyl carboxylic acid derivative
- Imidazole
- Acetamide
- Carboxamide group
- Primary carboxylic acid amide
- Secondary carboxylic acid amide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Sulfenyl compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organosulfur compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kcr-0983000000-9c514b8d51653caa1224 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06ur-1590000000-9e320bebf85c005c4822 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0059-3900000000-05782cb77ecb717613ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-2940000000-68f786d93bd3a77d6839 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01b9-9460000000-a4d709edaaed49aa33ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-80b8bbf76eaa18f0f047 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0298000000-fbed7b3e5fc06b678091 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01b9-0920000000-f52674108e45f179d8f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02t9-1920000000-18cf3dcfea935f5a560e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0290000000-8abadccd96fd80dc0926 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ukc-3900000000-80c53bf61586a9211df0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-80bd7a0de7ed9bc0732e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0301989 |
|---|
| FooDB ID | FDB001716 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 7846046 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|